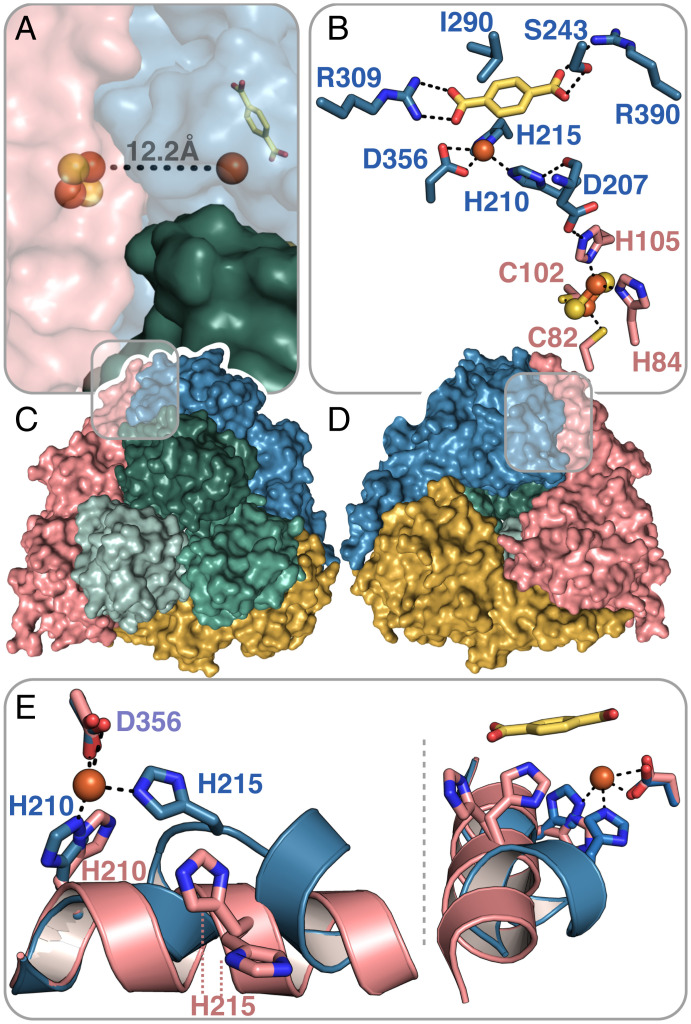
Fig. 4.
TPADO structure shows TPA binding interactions. (A and C) Top view of the α3β3-heterohexamer. The β-subunits are colored in shades of green, and the α-subunits are colored in pink, yellow, and blue. An iron–sulfur cluster and mononuclear iron cofactors are shown as spheres, exhibiting a head-to-tail intersubunit pathway for electron transfer. (B and D) The bottom view shows the trimeric architecture of the α-subunits. The bound TPA substrate is shown in yellow for the blue-colored α-subunit. Salt bridges with R309 and R390 and a hydrogen-bonding interaction with S243 are shown. The ferrous ion, which is coordinated by the side chains of residues H210, H215, and D356, is connected to the Rieske-type [2Fe-2S] cluster of a neighboring α-subunit (pink). (E) Superposition of two α-subunits of the TPA-bound structure shows two different conformations of an α-helix within the active site, which contains the ferrous ion-coordinating histidines H210 and H215. The pink α-helix is stabilized by crystal contacts, whereas the blue α-helix is kinked to allow the coordination of the ferrous ion by H215 when bound to TPA.