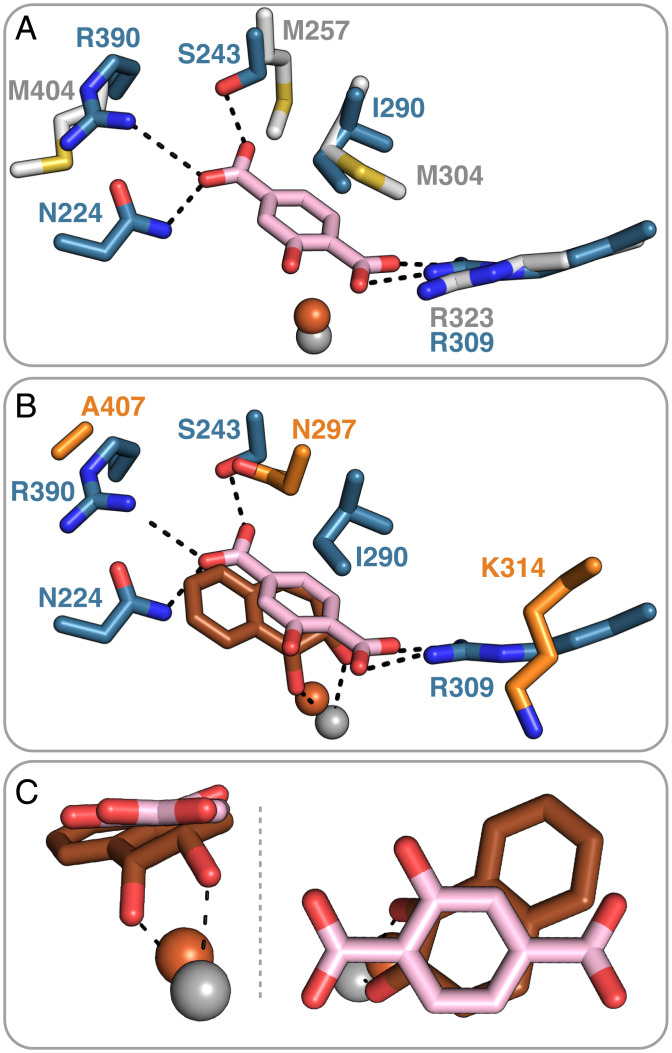
Fig. 5.
Active site structure of TPADO with 2-OH-TPA bound is shown superimposed on the structure of (A) NagGH and (B and C) product-bound NDO. (A) Superposition of the blue-colored TPADO α-subunit with the α-subunit of its closest structural homolog NagGH (gray), which has no substrate bound (PDB ID code 7C8Z), highlights the unique roles played by S243 and R390 in accommodating the substrate (38). (B) Superposition with the α-subunit of NDO (orange) with its dioxygenated product bound (PDB ID code 1O7P) (30). (C) Closer views of the overlay of the NDO product (orange) with 2-OH-TPA substrate and their relative positions to the ferrous ion. Overlap of the 1,2-diol portion of the NDO product with the ring 1,2-carbons of 2-OH-TPA suggests that these are the sites of hydroxylation.