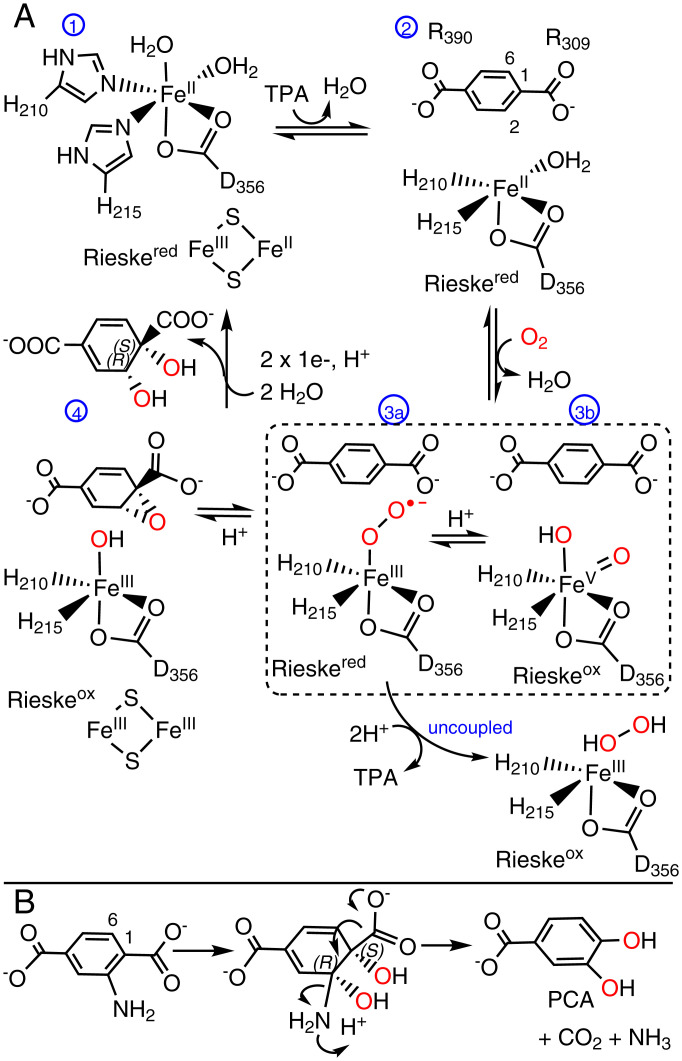
Scheme 1.
A canonical mechanism for ROs adapted for TPADO. (A) The fully reduced, resting active site (1) has a ferrous hexacoordinate mononuclear iron and reduced Rieske cluster (Rieskered). TPA binding (2) displaces a water molecule and primes the enzyme for a reaction with O2. The positions of residues involved in anchoring the substrate (R390, R309) are indicated. O2 binds in an end-on fashion to form (3a) the ferric-superoxy intermediate. One electron donation from the Rieske cluster forms a peroxy adduct, which can either heterolytically cleave to yield a high valent iron-oxo species (3b) or release H2O2 following diprotonation in the unproductive, uncoupling pathway (labeled uncoupled). A species resembling (3a) or (3b) (shown together in the dashed box) may react with TPA to form (4) an epoxide and ferric-hydroxy intermediate. The ferric-OH attacks the epoxide, which opens and protonates to form the product. Rereduction and hydration of the active site occur as the product departs, returning the active site to its starting state. The cis-1S,2R-DCD isomer is drawn as the presumptive product based on the observed products of the reactions between NADH/O2 and 2-NH2-TPA or 2-OH-TPA. (B) Hydroxylation of the ring 1,2-carbons of 2-NH2-TPA yields an intermediate analogous to DCD. Arrows show the pathway for rearomatization and loss of CO2 + NH3.