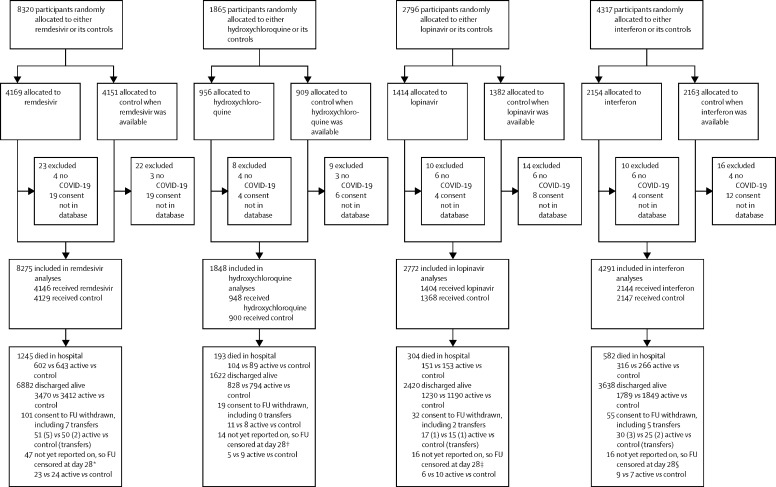
Figure 1.
Trial profile
14 304 hospital inpatients were randomly allocated (with equal probability) between the local standard of care (control group) and whichever of the four study drugs (active group) were locally available. 83 patients with a refuted COVID-19 diagnosis (all of whom survived) or with no encrypted image of their signed consent forwarded to the database were excluded, leaving 14 221 patients included. For each study drug, the control participants for that drug were those who could have been randomly allocated to receive it but were, by chance, randomly allocated to receive the same management without it. IFN=interferon. *Entry ended on Jan 29, 2021. †Entry ended on June 19, 2020. ‡Entry ended on July 4, 2020. §Entry ended on Oct 16, 2020.