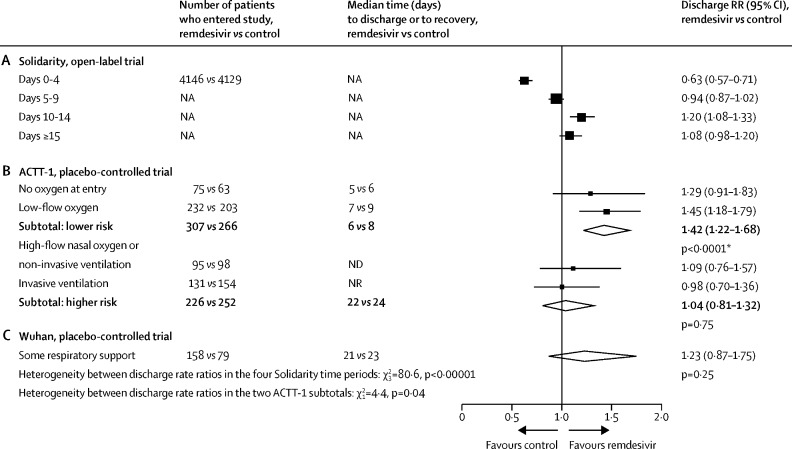
Figure 5.
Comparison between the effects of random allocation to remdesivir on the daily discharge rate in Solidarity and in two placebo-controlled trials
(A) Solidarity data are shown as treatment effects during different time periods. (B) ACTT-1 data are shown as treatment effects, split by initial respiratory support. (C) Wuhan data are shown as treatment effects among all patients. NA=not applicable. ND=not done. NR=median not reached. RR=rate ratio. *In ACTT-1, there was a chance imbalance favouring remdesivir in the initial proportions at higher risk, as defined in this figure (remdesivir: 226 [42%] of 533 vs placebo: 252 [49%] of 518, p=0·02). So, any findings combining low-risk and high-risk patients in that trial depend on whether this imbalance is allowed for. Cited ACTT-1 analyses are from the published text and its appendix.7