FIGURE 7.
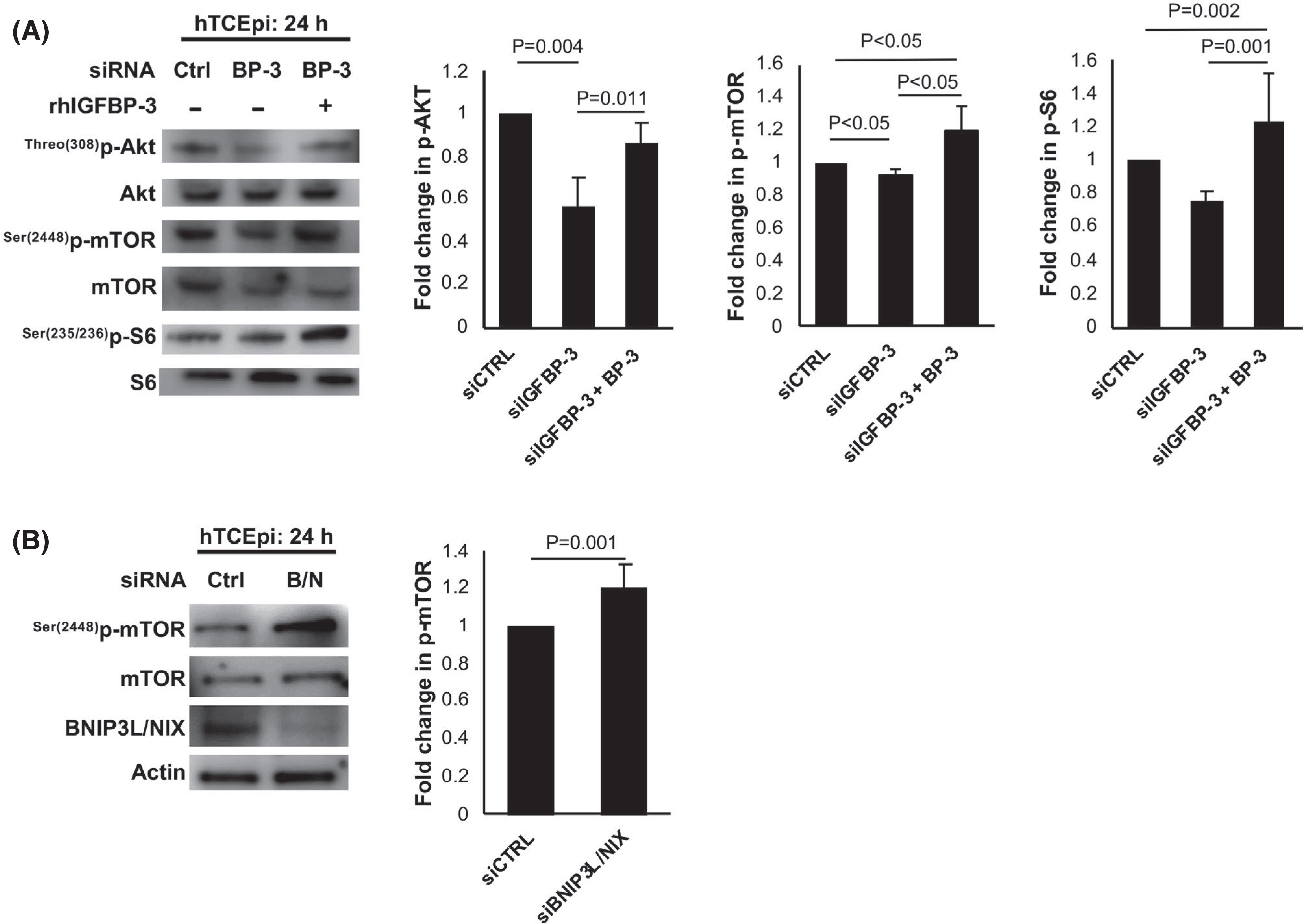
IGFBP-3 blocks mitophagy through activation of mTOR. (A) hTCEpi cells were transfected with siRNA oligonucleotides targeting IGFBP-3. Non-targeting oligonucleotides were used as a control. Cells were then cultured in KBM with or without 500 ng/ml rhIGFBP-3 for 24 h. Immunoblotting for phosphorylation of Akt, mTOR, and S6 proteins in hTCEpi cells. There was a decrease in phosphorylation of AKT (p = .004) and mTOR (p < .05) in knockdown treated cells. Treatment with rhIGFBP-3 increased phosphorylation of mTOR (p < .05 compared to knockdown and control), p-Akt (p = .011 compared to knockdown), and S6 (p = .001 and p = .002 compared to knockdown and control). All phosphorylated proteins were normalized to the respective total protein expression. (B) Cells were transfected with siRNA oligonucleotides targeting BNIP3L/NIX. Non-targeting oligonucleotides were used as a control. Cells were then cultured in KBM for 24 h. Knockdown was confirmed using immunoblotting. Immunoblotting for phosphorylation of mTOR was completed by normalizing phosphorylated protein to total protein expression. Knockdown of BNIP3L/NIX increased mTOR phosphorylation (p = .001). Data representative of mean ± standard deviation from three repeated experiments. One-way ANOVA with Student–Newman–Keuls post hoc multiple comparison test. KBM, keratinocyte basal media
