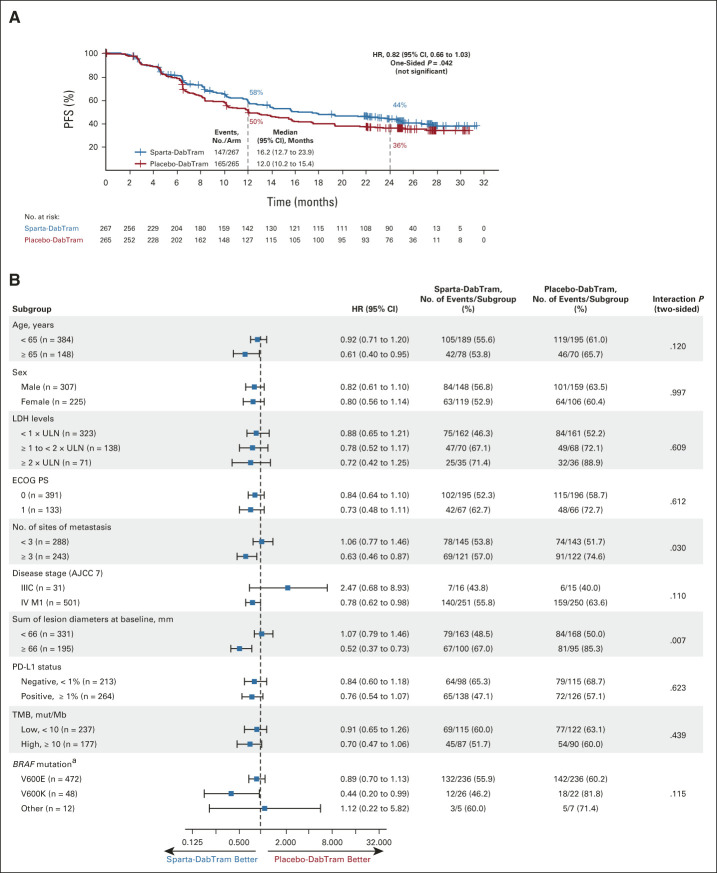
FIG 2.
(A) Kaplan-Meier estimates of investigator-assessed PFS in the intention-to-treat population and (B) analysis of PFS in predetermined prognostic subgroups. (A) The log-rank P value of .042 is one-sided and thus not significant. (B) P values are two-sided for treatment by subgroup interaction. aBRAF V600 mutation as determined by local testing. AJCC 7, American Joint Committee on Cancer's Cancer Staging Manual, 7th edition; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; mut/Mb, mutations per megabase; PD-L1, programmed death ligand 1; PFS, progression-free survival; placebo-DabTram, placebo plus dabrafenib and trametinib; sparta-DabTram, spartalizumab plus dabrafenib and trametinib; TMB, tumor mutational burden; ULN, upper limit of normal.