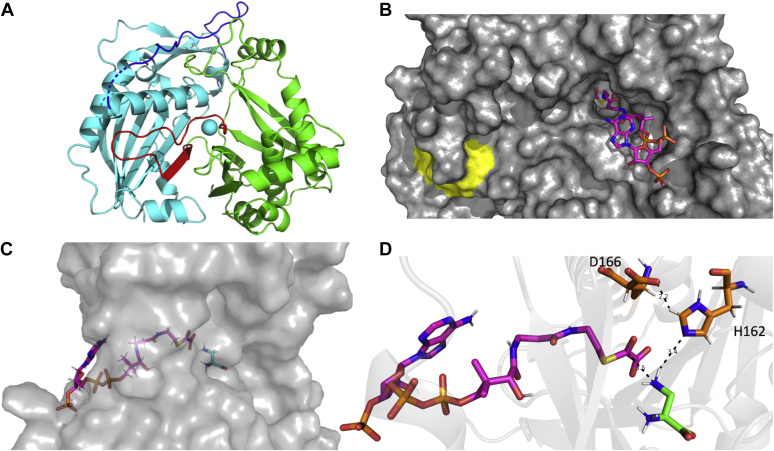
Figure 4.
Structure of BOS and docked substrates. A, ribbon diagram of BOS (PDB ID: 6ZBS). The N- (cyan) and C-terminal (green) domains are connected by loop (residues 183–209; blue) and segment comprised of a β-strand and a short loop (residues 371–393, red). B, surface representation with oxalyl-CoA (magenta) docked into the active site. The conserved DFGWG motif is colored yellow. C, side view showing both oxalyl-CoA (magenta) and L-DAPA (cyan) docked into the active site. D, a close-up view showing oxalyl-CoA (magenta) and L-DAPA (green) docked into the active site along with the conserved catalytic residues His162 and Asp166 (orange). A rotamer of Asp166 that places its residue in hydrogen-bonding distance to His166 was chosen manually to demonstrate a possible interaction. H-bonds are denoted by dashed lines. BOS, β-ODAP synthetase; L-DAPA, L-α,β-diaminopropionic acid.