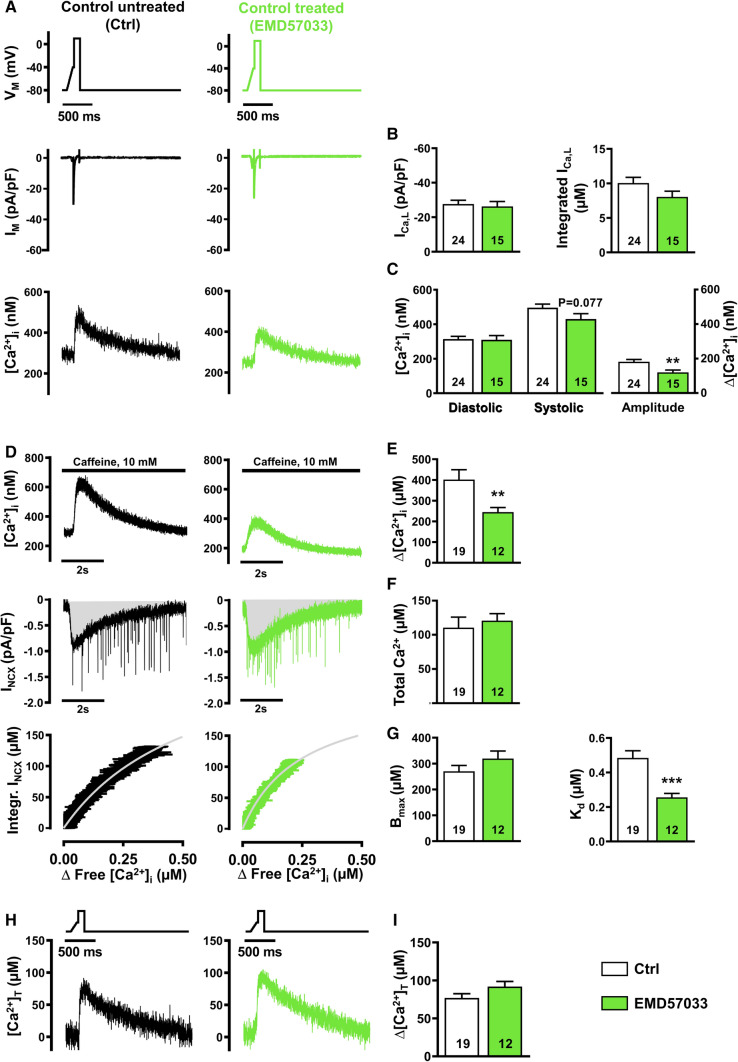
Fig. 5.
ICa,L and Ca2+ transient (upper), sarcoplasmic reticulum Ca2+ load and intracellular Ca2+ buffering (middle) and total cytosolic Ca2+ concentrations during ICa,L triggering (lower) in control (Ctrl) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) pre-treated with EMD57033 (5 µM). A Voltage-clamp protocol (upper), representative simultaneous recordings of ICa,L (middle) and corresponding ICa,L-triggered Ca2+ transients (CaT, lower) in untreated Ctrl iPSC-CM (left) and Ctrl iPSC-CM pre-treated with EMD57033 (right). B Peak ICa,L amplitude (left) and integrated ICa,L (right). C Diastolic and systolic [Ca2+]i (left) and Ca2+ transient amplitude (right). D Representative recordings of caffeine-induced Ca2+ transient i.e. free cytosolic Ca2+ concentration (upper) with associated depolarising inward current (INCX, middle) in untreated Ctrl iPSC-CM (left) and Ctrl iPSC-CM pre-treated with EMD57033 (right). Integrated INCX as an index for total cytosolic Ca2+ concentration was plotted against corresponding cytosolic free Ca2+ concentration (lower). Buffer curves depicting the relationship between cytosolic free and total Ca2+ were fitted with hyperbolic functions. E, F Sarcoplasmic reticulum Ca2+, quantified with caffeine-induced Ca2+ transient amplitude (E), or area under the curve (Integral) of the corresponding inward current (INCX) (F). G Maximum buffer capacity (Bmax, left) and dissociation constant (Kd, right), determined from buffer curves. H Representative total cytosolic Ca2+ concentration during ICa,L-triggered Ca2+ transients in untreated Ctrl iPSC-CM (left) and Ctrl iPSC-CM pre-treated with EMD57033 (right). I Total cytosolic Ca2+ amplitude. n = number of iPSC-CM from three batches. Data are mean ± SEM. **P < 0.01 and ***P < 0.001 vs. Ctrl using Student’s t test (B, C left, F, G, I) and the Mann–Whitney U test (C right, E)