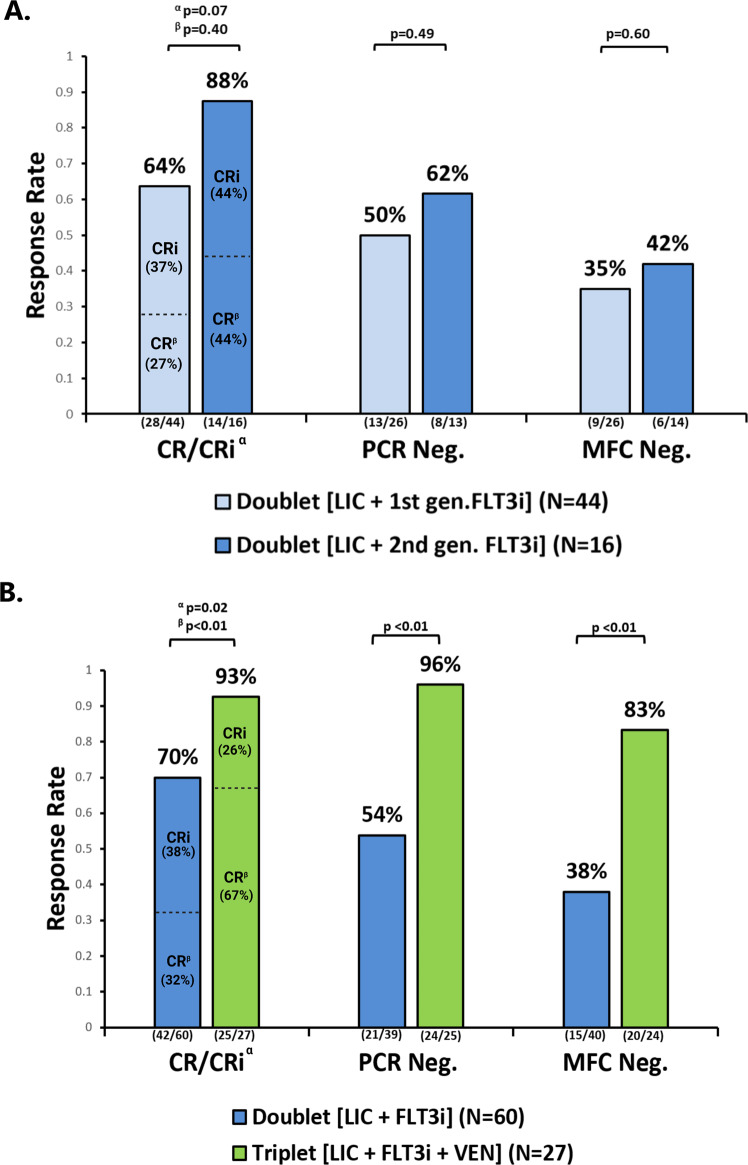
Fig. 2. Comparison of remission rates and measurable residual disease (MRD) negativity rates in patients treated with 1st generation FLT3i doublets vs. 2nd generation FLT3i doublets (A) and doublets vs. triplets (B).
A. Of the 60 patients treated with the doublet regimens, 44 (73%) received a first-generation FLT3i and 16 (27%) a second-generation FLT3i. We noted no statistically significant difference in CR/CRi (64% versus 88%, P = 0.07α),CR (27% versus 44%, P = 0.40β), FLT3 PCR (50% versus 62%, P = 0.49) or MFC negativity (35% versus 42%, P = 0.60) rates in patients treated with first- (n = 44) or second-generation (n = 16) FLT3i based doublets. Both PCR and MFC testing were missing in 2 responders in group treated with a 1st generation FLT3 inhibitor. PCR and MFC testing were missing in 3 and 2 responders, respectively, in group received a 2nd generation FLT3 inhibitor. B The rates of CR/CRi (93 versus 70%, P = 0.02α), FLT3 PCR negativity (96% versus 54%, P < 0.01), and MFC negativity (83% versus 38%, P < 0.01) were significantly higher with the triplet regimen than with the doublet regimen. Triplet regimens not only resulted in higher CR/CRi rates, but importantly in a meaningfully higher true CR rates (67% versus 32%, P < 0.01β). PCR and MFC testing were missing in 3 and 2 responders, respectively in doublet arm. MFC testing was missing in 1 responder in triplet arm. MRD assessments were performed by multicolor flow cytometry (MFC, sensitivity of 10−3) and multiplex polymerase chain reaction (FLT3-PCR, sensitivity of 10−2) for ITD and kinase domain (D835). LIC low intensity chemotherapy, FLT3i FLT3 inhibitor, VEN venetoclax; CR/CRi complete response/complete response with incomplete count recovery, PCR RT-polymerase chain reaction assay for FLT3; MFC multicolor flow cytometry; Neg., negative.