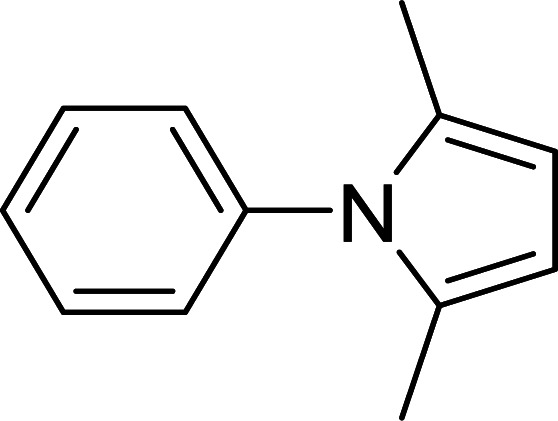
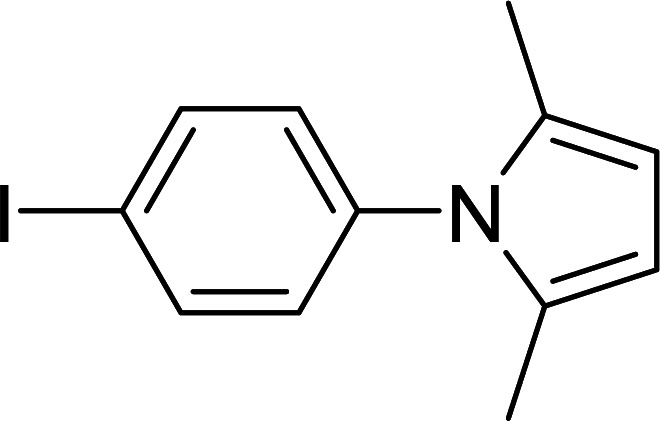
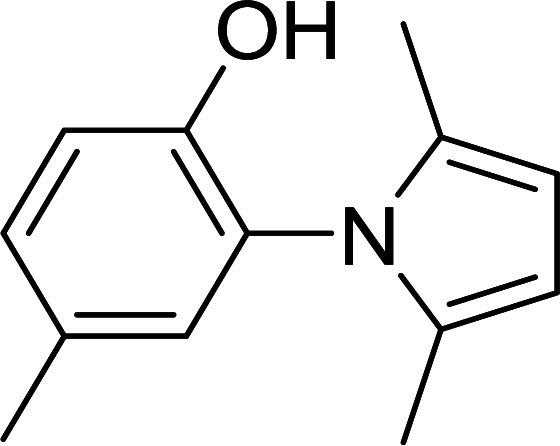
Synthesis of pyrroles catalyzed by MIL-53(Al)a.
| Entry | Amine | Time (min) | Product | Isolated yieldb (%) |
|---|---|---|---|---|
| 1 |

|
15 |
|
96 |
| 2 |

|
20 |

|
86 |
| 3 |

|
30 |

|
85 |
| 4 |

|
30 |

|
88 |
| 5 |

|
30 |

|
80 |
| 6 |

|
15 |
|
96 |
| 7 |

|
15 |

|
98 |
| 8 |

|
15 |

|
95 |
| 9 |

|
15 |

|
98 |
| 10 |

|
15 |
|
98 |
| 11 |

|
15 |

|
75c |
| 12 |

|
20 |

|
90 |
| 13 |

|
15 |

|
98 |
| 14 |

|
15 |

|
95 |
| 15 |

|
15 |

|
98 |
| 16 |

|
15 |

|
50 |
| 17 |

|
20 |

|
Trace |
| 18 |

|
15 |

|
55 (92)d |
The reaction conditions: amines (1 mmol), acetonylacetone (1.2 mmol).
Isolated yield.
Yield of another isomer was obtained in 15%.
Yield in parenthesis was reported by the reaction with 2.4 equiv. of acetonylacetone.
