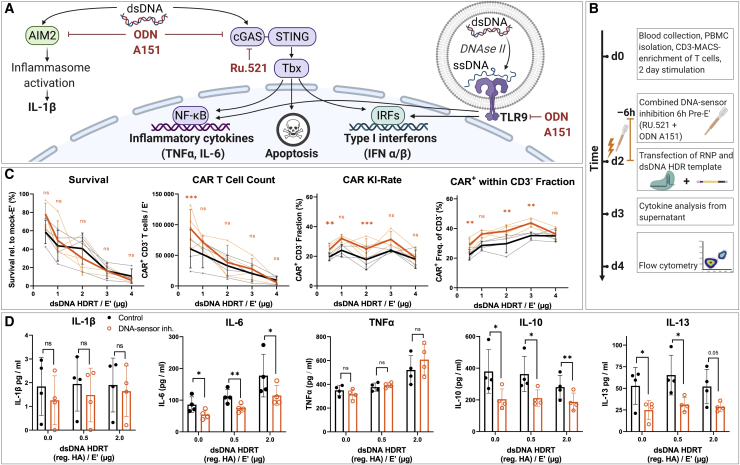
Figure 2.
DNA-sensor inhibition increases relative CAR insertion rate with minimal improvement of survival at optimized dsDNA donor dosage
(A) Illustration of common DNA-sensing pathways (in immune cells) that induce downstream cytokine production and the presumed mode of action of different DNA sensor inhibitors used in subsequent experiments. (B) Experimental setup for the combined addition of TLR-9 inhibitor ODN A151 and the cGAS-inhibitor RU.521 with escalating amounts of dsDNA donor templates is shown (as in Figure 1B). DNA sensor inhibitors were supplemented together into the medium 6 h prior to co-electroporation of RNP and reg. HA dsDNA donor templates. (C) Summary of flow cytometric analysis 2 days after electroporation. Data were obtained in parallel to controls presented in Figures 1D–1G (n = 4 healthy donors in two independent experiments). Editing outcomes of T cells that received combined DNA-sensor inhibition prior to electroporation are shown in orange. Black indicates the control values from Figure 1E. Thick lines indicate mean values; error bars indicate standard deviation. Light dots represent individual values. Light lines connect these for each donor. Descriptive statistical analysis was performed using paired, two-tailed Student’s t tests comparing values for DNA-sensor inhibition with values for no intervention. (D) Summary of supernatant analysis 24 h post-electroporation for cytokines associated with DNA sensing: IL-1β (lower limit of detection [LOD]: 0.85 pg/mL), IL-6 (lower LOD: 0.13 pg/mL), and TNF-α (lower LOD: 0.05 pg/mL) as well as the Th2-associated cytokines IL-10 (lower LOD: 0.01 pg/mL) and IL-13 (lower LOD: 0.27 pg/mL). Descriptive statistical analysis was performed as for (C).