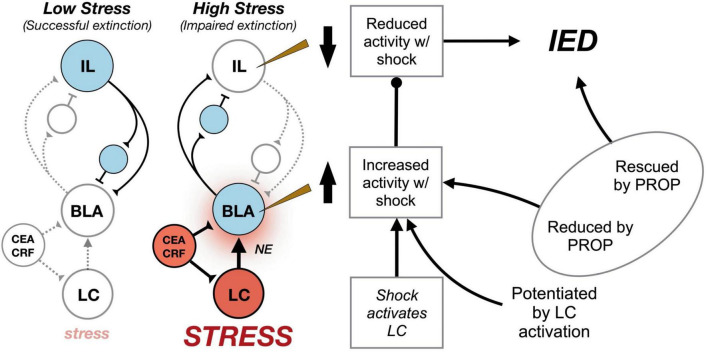
FIGURE 2.
Circuit model for the immediate extinction deficit (IED). Under basal conditions (“Low Stress”) delayed extinction procedures conducted 24 h after fear conditioning recruit infralimbic (IL) cortical circuits (blue) that mediate the acquisition and expression of extinction learning. Inhibition of conditioned fear is presumed to arise from IL-mediated excitation of inhibitory interneurons (small circles) that reduce the excitability of basolateral amygdala (BLA) principal cells representing fear memories. However, delivering extinction trials soon after fear conditioning, when animals are under extreme stress (“High Stress”) results in activation of locus coeruleus (LC) noradrenergic neurons (red) that release norepinephrine (NE) in forebrain targets, including the BLA (blue). Neurons in the central amygdala (CEA) release corticotropin-releasing hormone (CRF) in the LC and BLA to facilitate stress-induced activation of these brain areas. Consequently, fear conditioning dramatically increases spontaneous spike firing in the BLA, while decreasing spike firing in IL. We speculate that BLA decreases IL spike firing by activating IL interneurons (small circles) and driving feed-forward inhibition in IL principal cells. Shock-induced increases in BLA firing are modulated by the LC, and LC activation during weak shock enables the IED when it would not normally occur. Systemic or intra-BLA administration of the β-adrenergic antagonist, propranolol (PROP), attenuates the IED and limits shock-elicited changes in BLA and IL spike firing (Fitzgerald et al., 2015; Giustino et al., 2016a,2017, 2020).