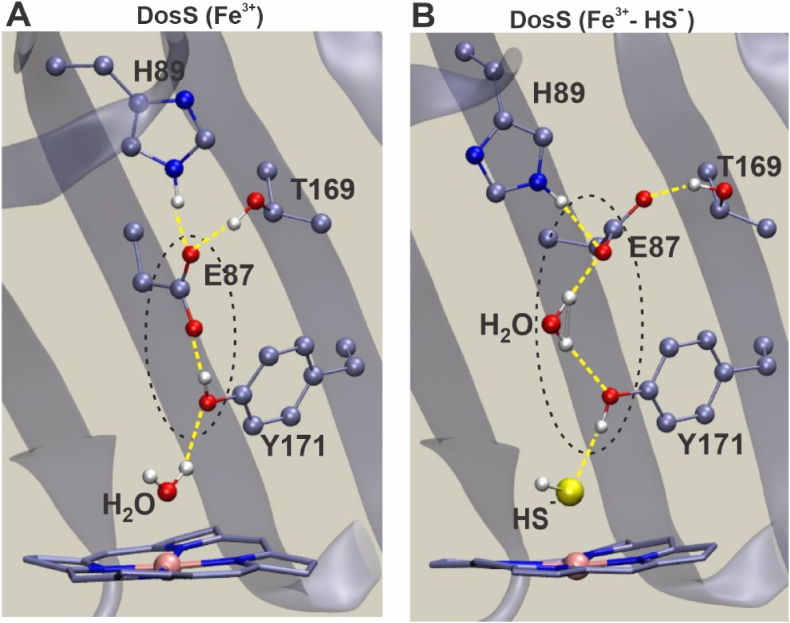
Fig. 5.
Sulfide binding alters the hydrogen bonding network in the DosS heme pocket (A) MD-modeled hydrogen bonding network in the distal domain of ferric (Fe3+) DosS showing an intact H-bond between glutamate (E87) and tyrosine (Y171). (B) MD-modeled hydrogen bonding network in the distal domain of ferric (Fe3+) DosS in the presence of sulfide showing disrupted H-bonding between glutamate (E87) and tyrosine (Y171). Predicted changes in the H-bonding patterns may lead to structural changes which alter DosS kinase activity upon sulfide binding.