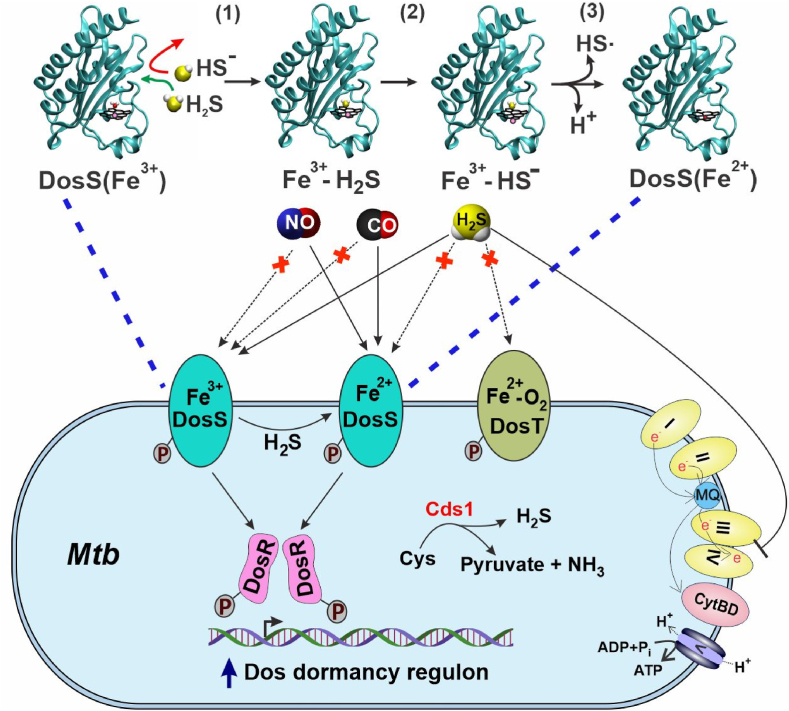
Fig. 7.
Schematic of a proposed mechanism of H2S sensing by Mtb. H2S derived from the host or by Mtb via Cysteine desulfhydrase 1 (Cds1, Rv3684) can enter the DosS heme pocket (green arrow). (1) Based on sMD modeling, the charged HS− species does not enter the heme pocket (red arrow). Upon entering the heme pocket, H2S binds to the heme iron of DosS(Fe3+) only, demonstrating that sulfide sensing is different than for NO and CO, which bind only DosS(Fe2+). In contrast, DosT does not bind H2S. (2) QM/MM MD simulations suggest that H2S deprotonates to form bound hydrosulfide anion (HS−). The proton acceptor is predicted to be a water molecule located opposite from the distal Tyr (i.e., close to the solvent-exposed heme edge) which then subsequently transfers a proton to the heme propionate. (3) The heme propionate donates a H2S-derived proton to the solvent and sulfide can slowly reduce the DosS heme iron, followed by the release of a hydrosulfide radical (HS.) to the solvent. DosS(Fe2+) can then bind CO or NO. DosS autokinase activity is lowest for unbound DosS(Fe3+) and is increased via binding of H2S to DosS(Fe3+) or reduction to DosS(Fe2+), which could ultimately lead to increased expression of DosR dormancy regulon genes. At higher concentrations, H2S inhibits Complex IV, resulting in rerouting of electrons through cytochrome bd oxidase (CytBD). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)