Figure 2. Patient Response to Treatment From Prerandomization During the Combined Titration and Maintenance Period and/or Maintenance Only Period.
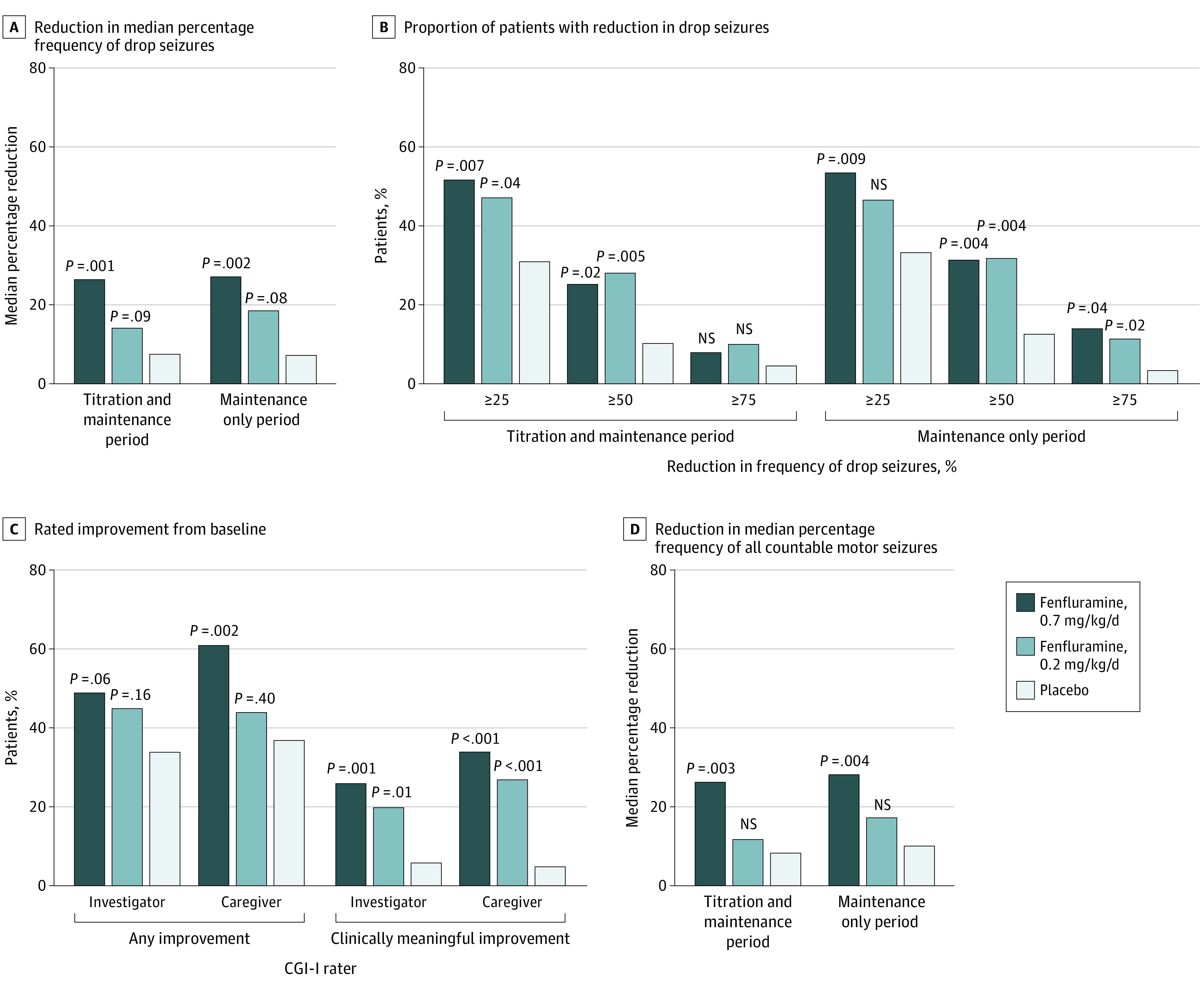
A, Estimated median difference from placebo was calculated using Hodges-Lehmann estimate. B, The 50%, 25%, and 75% or greater responder levels were included. C, P values were calculated using the Cochran-Mantel-Haenszel test. D, Countable motor seizures included generalized tonic-clonic (GTC) seizure, secondary GTC, tonic seizure, atonic seizure, tonic or atonic seizure, clonic seizure, hemiclonic seizure, and focal seizure. P values were statistically significant for the primary efficacy outcome and the 50% or greater responder rate for the 0.7-mg/kg/d fenfluramine group; all other P values were nominal. Distribution data are presented in eFigure 2 in the Supplement. CGI-I indicates Clinical Global Impression-Improvement scale; NS, not statistically significant.
