Figure 3. The soluble domain of NDM-1 is optimized to interact with the outer bacterial membrane.
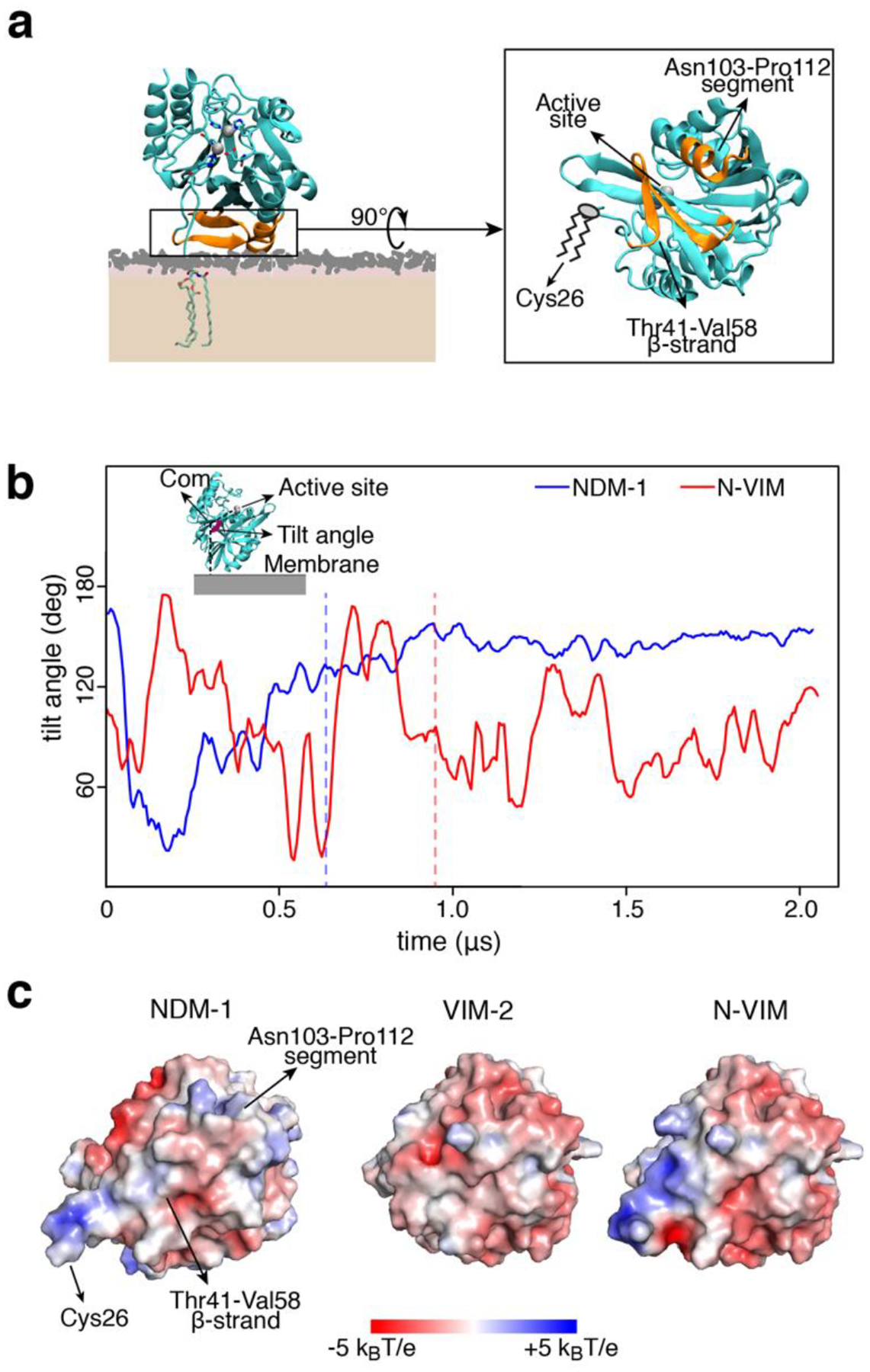
(a) Final and stable orientation of NDM-1 with respect to the membrane, and protein domains that are involved. The active site is exposed to the solvent and not occluded by the membrane. (b) Tilt angle definition and evolution of the tilt angle vs. time, for NDM-1 and N-VIM at the outer bacterial membrane. The tilt angle is defined as the angle formed by the active site, the center of mass of the protein and the projection of the center of mass of the protein on the plane of the membrane. The vertical dashed lines represent the moment in which the anchoring occurs. (c) Electrostatic potential of the protein-membrane surface of interaction (seen from the membrane surface) for NDM-1, VIM-2 and N-VIM.
