Figure 37.
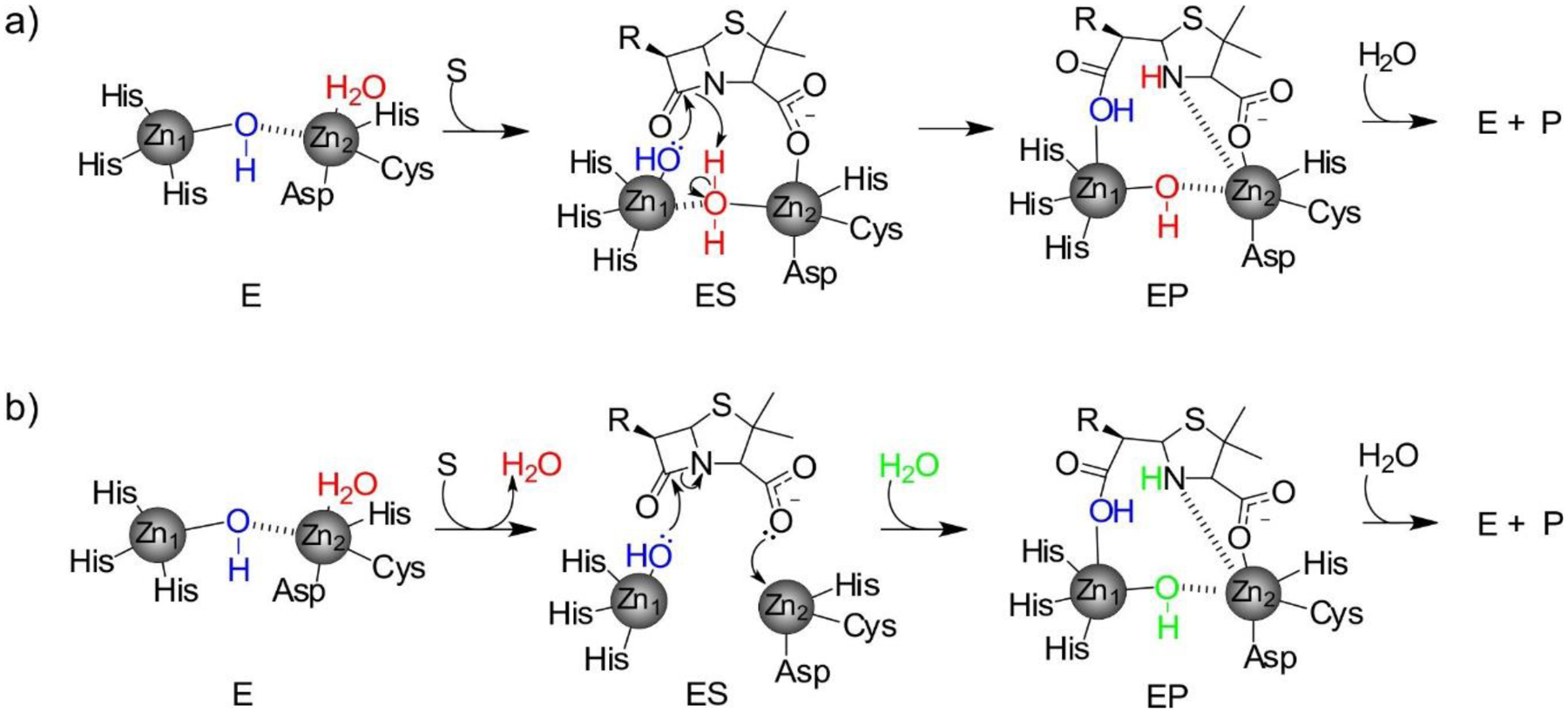
Mechanism of penicillin hydrolysis by bi-Zn(II) MBLs, with two proposed pathways for the protonation step. a) The nucleophilic hydroxide (blue) detaches from Zn2 upon substrate binding in ES. The water ligand originally bound to Zn2 (red) becomes a bridging ligand, becoming acidic and acting as the proton donor and regenerating the nucleophilic hydroxide. Based on the work from Llarrull, Vila and coworkers287. b) The Zn2-bound apical water (red) is detached from the metal site upon substrate binding. An additional water molecule from the bulk solvent (green) is incorporated into the active site in the subsequent step, acting as the proton donor. Based on the work from Nair and coworkers.244
