Figure 70.
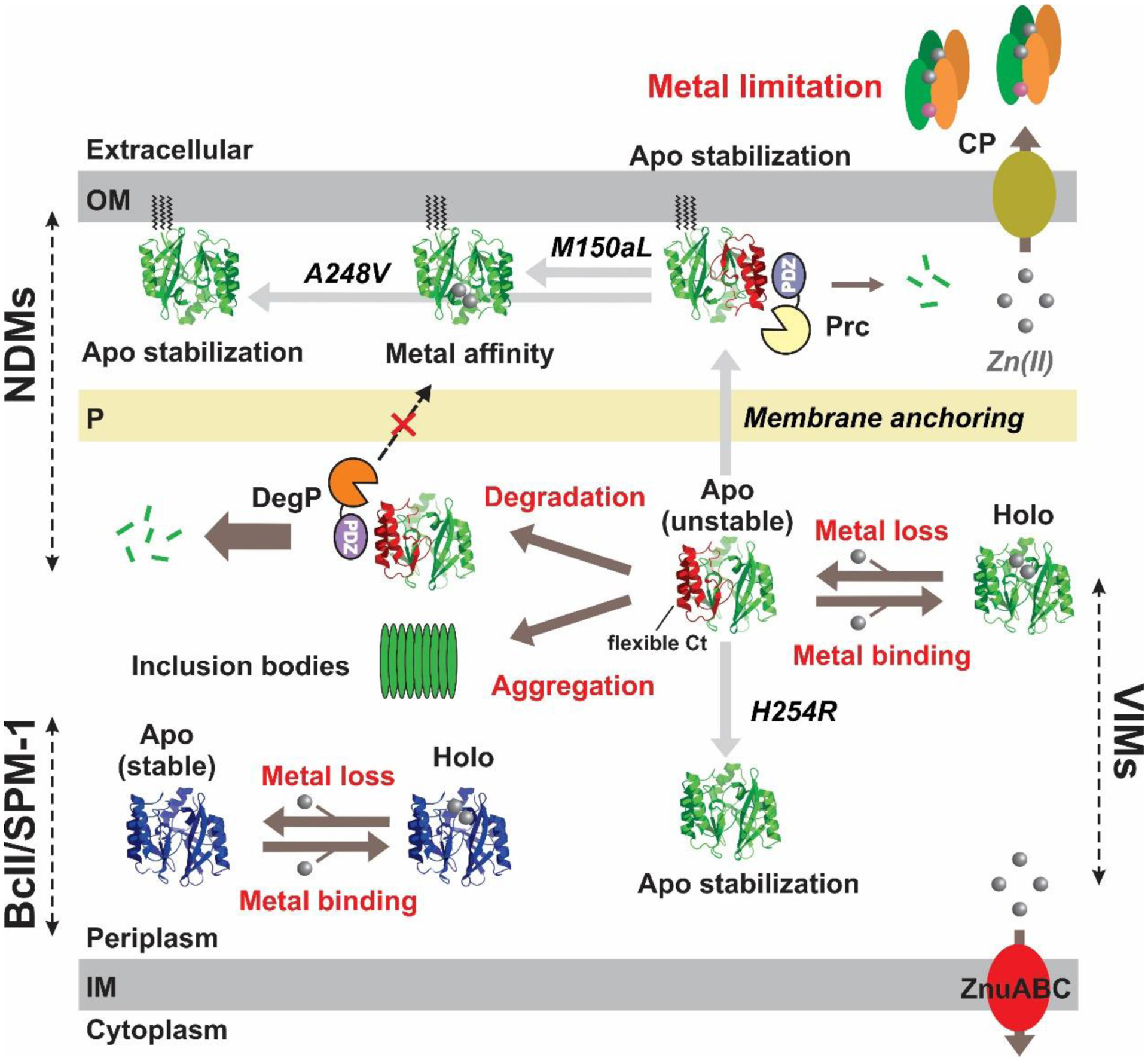
Model of zinc-dependent modulation of MBL stability in vivo for NDMs, VIMs, BcII and SPM-1. Apo enzymes generated during metal limitation are susceptible to degradation and aggregation. Anchoring of NDM-1 to the outer membrane prevents degradation by DegP due to the permeability barrier of the peptidoglycan layer preventing passage of this high molecular weight protease. Mutations H254R (in VIM-2) and M150aL and A248V (in NDM-1) improve tolerance of these MBLs to Zn(II) depletion either by improving stability of the apo enzymes or by increasing their Zn(II) affinity.
