Figure 1.
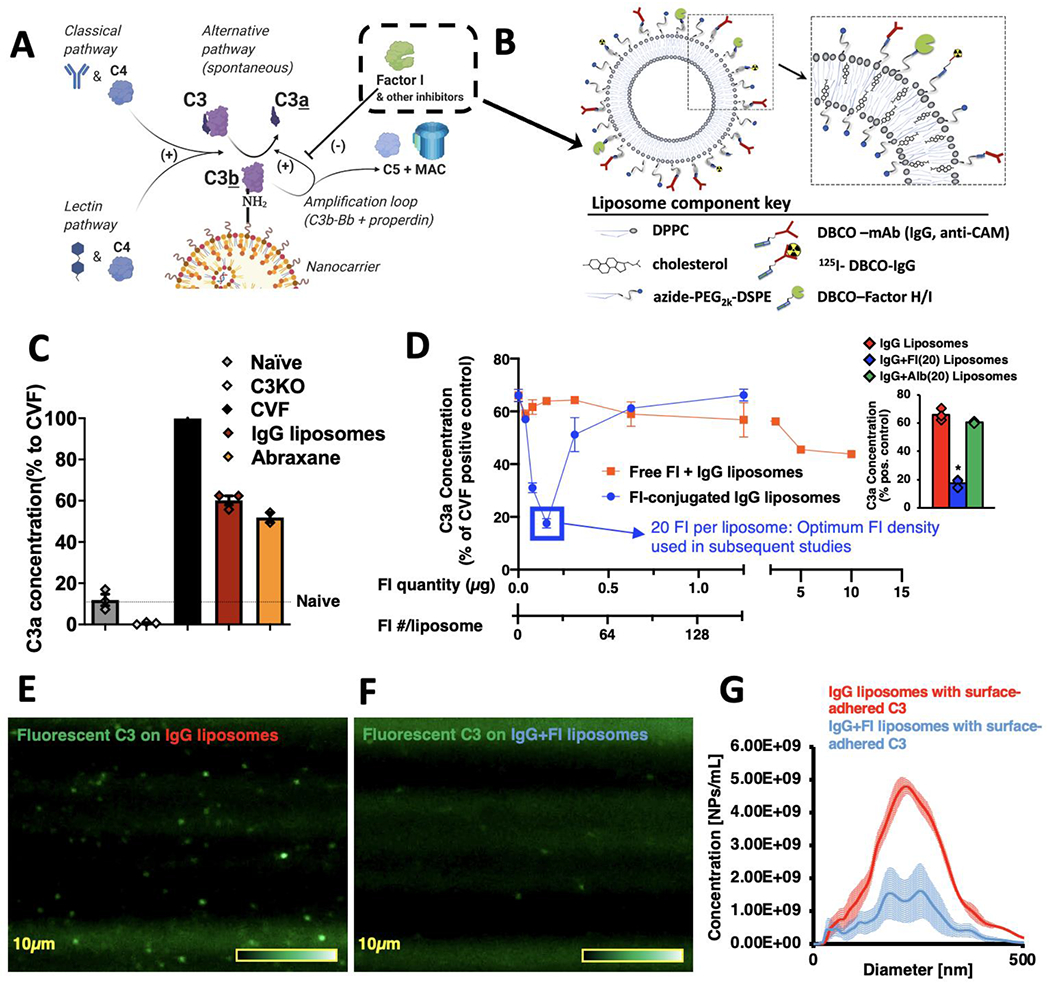
Conjugation of Factors I to nanoparticles inhibits C3-opsonization in vitro. a) Diagram of the reactions leading to C3 adducts forming on the surface of nanoparticles. The reactive thioester of C3 attacks surface nucleophiles, such as amines, resulting in a covalent C3b-surface adduct, and releasing the small protein C3a. This slow C3-surface reaction is catalytically accelerated by enzymes of the classical, lectin, and alternative pathways. C3b-surface adducts form a complex (C3bBb) that catalyzes further C3b to deposit on adjacent nucleophiles (the “amplification loop”), which allows C3b to rapidly spread across a surface. On mammalian cell surfaces, C3b is rapidly broken down by nearby regulators of complement activity (RCAs), such as Factor I, breaking the amplification loop and preventing formation of the most potent anaphylatoxin, C5a. b) Schematic of the nanoparticles on which we tested the effects of Factors H & I conjugation. All surface proteins were first conjugated to DBCO via its NHS ester, and then were conjugated onto the surface of liposomes by cycloaddition with azides on the end of the liposomes’ phospholipid DSPE-PEG2k-azide. c) Clinically used nanoparticles induce complement activation in vitro. Into mouse plasma, we mixed either Abraxane (nab-paclitaxel) or liposomes conjugated to random IgG molecules (to represent nanoparticles conjugated to targeting moieties). Reactions were EDTA-quenched after 10 minutes, and C3-nanoparticle adduct formation was measured indirectly by C3a ELISA. Data is presented as the % of C3a compared to the positive control, cobra venom factor (CVF), which rapidly cleaves all C3 in solution. Compared to naive serum (no nanoparticles), both nanoparticles induced large amounts of C3a. d) Factor-I-conjugation to nanoparticles efficiently reduces C3a production. Onto IgG-liposomes, we conjugated varying amounts of Factor I (blue trace), incubated in serum using the protocol of (c), and measured C3a production. In separate experiments, we incubated IgG-liposomes with free Factor I (not conjugated to the nanoparticles; red trace). Conjugating 20 Factor I molecules onto the liposomes reduced C3a production 3.7-fold (blue box), while free Factor I molecules at a dose equivalent to 1280 Factor I per liposome led to a 27% reduction in C3a. (n=3, per condition). Inset: Comparison of C3a production by IgG liposomes with 20 Factor I per liposome (blue) vs. C3a production by IgG liposomes without Factor I or vs. C3a production by IgG liposomes with 20 albumin per liposome. Factor I induced a 73.4% reduction in C3a vs. IgG alone and a 71.0% reduction in C3a vs. irrelevant protein. e) Direct measurement of C3-nanoparticle surface adducts. Purified C3 was conjugated to Alexa-488, added to serum, and then nanoparticles (IgG-liposomes) were added to allow opsonization. This mixture was then flowed through a Malvern Nanosight to image and size individual nanoparticles that had become bound to fluorescent C3. f) The same process as (e), but for Factor I-conjugated IgG-liposomes, showing far fewer fluorescent-C3 labeled nanoparticles. g) Histogram of the data from (e) & (f), showing Factor I conjugation leads to far fewer fluorescent-C3-opsonized nanoparticles. Statistics: For (d), inset: n=3; * = p=2.23x10−6 for comparison of IgG+FI(20) liposomes vs. IgG liposomes, p=4.43x10−6 for comparison of IgG+FI(20) liposomes vs. IgG+Albumin(20) liposomes.
