Figure 1. Adaptive Glutamine Catabolism following mTOR Inhibition in Lung SCC.
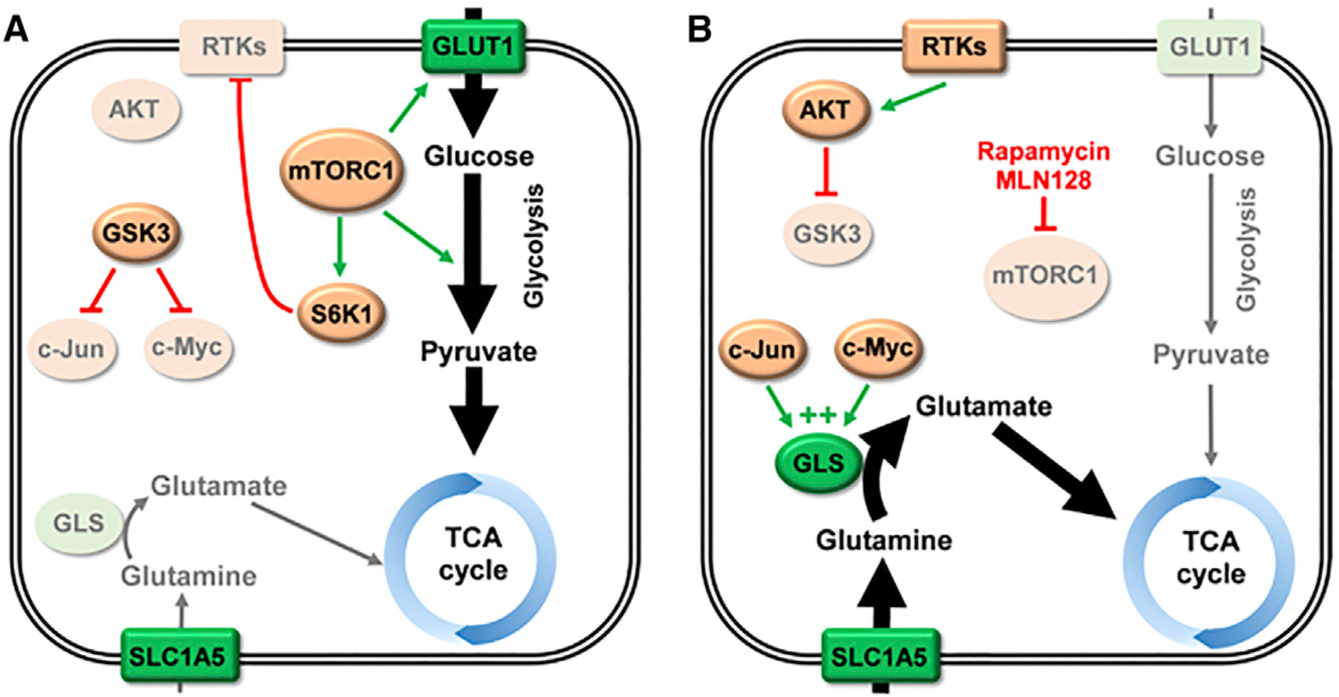
(A) In untreated lung squamous cell carcinoma (SCC), mTORC1 is often hyperactivated. One signaling axis downstream of mTORC1 leads to upregulated glucose uptake and glycolytic flux. A proportion of the glucose-derived carbon supplies the tricarboxylic acid (TCA) cycle. Another signaling axis activates S6K1, which mediates feedback inhibition of receptor tyrosine kinases (RTKs) such as the insulin receptor. Under these conditions, GSK3 is active and targets c-Myc and c-Jun for degradation, leading to decreased expression of glutaminase (GLS). (B) Treatment with the mTORC1 inhibitor rapamycin, or the mTOR kinase inhibitor MLN128, decreases glucose uptake, glycolytic enzyme expression, and supply of glucose-derived carbon to the TCA cycle. It also blocks activation of S6K1, and, consequently, feedback inhibition of RTKs is lost. Elevated RTK signaling activates AKT via phosphorylation of residue T308. Activated AKT then phosphorylates and inhibits GSK3, allowing c-Myc and c-Jun to accumulate. These transcription factors upregulate expression of GLS, increasing cellular glutaminase activity and allowing glutamine-derived carbon to supply the TCA cycle. Lighter shading indicates enzymes or pathways with suppressed expression/activity. Nutrient transporters and metabolic enzymes are shown in green. Abbreviations: GLS, glutaminase; GLUT1, glucose transporter 1; GSK3, glycogen synthase kinase 3; RTKs, receptor tyrosine kinases; TCA, tricarboxylic acid.
