Abstract
Anti-adeno-associated viral vector (AAV) neutralizing antibodies (NAbs) can ablate efficacy of transgene expression following intravenous vector administration. This observation in both preclinical and clinical trials has led to exclusion of NAb-positive patients from receiving AAV gene therapy. AAV drug development includes selection of capsids with lower NAb seroprevalence that also possess other favorable traits. Often a limited number of healthy volunteers are screened to gauge NAb seroprevalence. However, limited data sets can be biased leading to inaccurate estimates of NAb incidence. In this study, we evaluated AAV NAbs against a panel of vectors among healthy donors within the United States. While the overall seroprevalence against most AAVs was lower, we did observe increased NAb incidence among black and Hispanic donors. These findings of increased NAb seroprevalence among the minority races were confirmed in a second set of donors who also demonstrated higher seroprevalence among these races. Interracial- and intraracial differences within genders were also observed among donors. The increased incidence of AAV NAb among racial minorities was unexpected. Our findings underscore the need for removing bias in sample data sets and evaluating seroprevalence within the patient population while selecting capsids.
Keywords: AAV, neutralizing antibodies, race, seroprevalence
Introduction
Adeno-associated viral vectors (AAV) have emerged as the vector of choice to treat rare metabolic and neuro-muscular genetic disorders. These include three approved drugs to treat dyslipidemia, retinal blindness and spinal muscular atrophy.1 The surge in ongoing AAV clinical trials is a result of successes observed with these approved therapeutics and efficacy data from several ongoing clinical trials.2 AAV drug development relies on selection of an appropriate AAV capsid with desired traits for transgene delivery. The choice of vector is made after integrating data on vector tropism, biodistribution, safety, and manufacturability as part of the preclinical drug development process.3 Vectors with desired properties may still be deselected if they have higher neutralizing antibody (NAb) seroprevalence.
A higher NAb seroprevalence restricts the patients who can receive AAV gene therapy. The basis for excluding NAb positive patients was the inability to treat animals harboring preexisting NAb with AAV vectors.4 Similar findings were later reported in a clinical trial for hemophilia B where a patient with low level NAb failed to achieve transgene expression.5 Vector administration to NAb-positive patients also carries the risk of systemic inflammatory responses from immune complex formation, complement activation, detargeting to secondary lymphoid organs, and enhanced uptake into antigen presenting cells.6,7 Evaluation of anti-AAV NAb seroprevalence in subjects is an important first step that helps derisk selection of capsids with higher NAb seroprevalence.
Epidemiological studies have reported higher AAV seroprevalence in Africa compared to Europe, Australia, and the Americas. In a separate study, <40% United Kingdom adults were NAb positive against an engineered capsid AAV.LK03.8 In contrast, 90% of Chinese subjects were positive against AAV.LK03.9 During pilot studies, we became aware of skewed AAV NAb seroprevalence among donors in the United States.
To better understand the source of these differences, we screened a subset of donors for presence of NAb against a panel of AAV vectors. Our data demonstrate higher AAV NAb incidence among minority black and Hispanic subjects compared to whites, against multiple AAVs. Interracial seroprevalence differ more among males compared to females, and intraracial gender differences were also observed in some races. These findings expose a previously underappreciated heterogeneity in AAV NAb incidence by race that can impact seroprevalence estimates.
Materials and Methods
Donor serum
All serum samples were obtained from BIOIVT. Donors were healthy and self-identified as belonging to a race. There were nearly equal numbers of donors from each race (34 blacks, 32 whites, and 34 Hispanics) in cohort 1. Within each race group, there were equal numbers of men and women donors who varied in age from 20 to 80. There were more donors in the third, fourth, and fifth decade of life (18, 24, and 28 donors, respectively) compared to those aged younger than 30 years or older than 60 years (13 and 15 donors, respectively). There were only two donors aged >70.
Cohort 2 included an additional subset of 300 donors distributed equally across the three major races. Cohort 2 also had equal numbers of men and women donors who were distributed across all age groups. We did not have any donors aged >70 in cohort 2. Eighty-eight donors in cohort 1 were from the state of Florida, while 12 donors were from Pennsylvania within the United States. The 300 donors in cohort 2 were all from Florida.
AAV vectors
AAV1, AAV3B, AAV5, AAV9, AAVrh74, AAVDJ, and Spark100 vectors expressed Firefly luciferase (FLUC) from a ubiquitous promoter. Except Spark100, all vectors were obtained from the Viral Vector Core Facility at UMASS. Spark100 was produced at the Rare Disease Research Unit, Pfizer, Cambridge, MA following triple transfection of adherent HEK293 cells grown in HYPERFlasks (Corning). In brief, cell lysates and supernatant were clarified and tangential flow filtration concentrated before subjecting to Iodixanol gradient centrifugation. Isolated fractions were dialyzed, tittered, and stored at −80°C in PBS/5% glycerol before use.
NAb assay
A cell-based transduction inhibition assay was used to evaluate NAb. In brief, heat-inactivated donor serum diluted two-fold (5, 10, 20, and 40) was admixed with AAV vectors expressing firefly luciferase for an hour at 37 degrees before adding to HEK293 cells. Twenty-four hours later, Luciferase expression was quantified using Bright-Glo™ Luciferase Assay System (Promega). Plates were read on EnVision (Perkin Elmer). NAb titers are reported as the lowest reciprocal serum dilution that demonstrated ≥50% transduction inhibition.
Binding antibody assay
A modified enzyme-linked immunosorbent assay (ELISA)-based assay was used to quantify anti-AAV-binding antibodies (BAbs). Vector-coated plates were first prepared by adding 2 × 109 GC of vector per well. Plates were washed and blocked with 5% nonfat dry milk (Thermo Fisher Scientific) before adding donor serum diluted 1/100 in PBS. Bound antibodies were detected using a secondary goat-anti human IgG secondary antibody (Thermo Fisher Scientific) and quantified using a plate reader (SpectraMax i3x).
Statistical analysis
Statistical analysis was conducted using R version 4.0.2. The demographically corrected (weighted) estimated NAb seroprevalence in the United States population was calculated using the following weights: 0.653 (60.1%/92%) for white, 0.146 (13.4%/92%) for black, and 0.201(18.5%/92%) for Hispanic or Latino. According to 2019 U.S. census (https://www.census.gov/quickfacts/fact/table/US/PST045219), 60.1%, 13.4%, and 18.5% are the corresponding population percentage in United States for white, black, and Hispanic or Latino population, 92% is the sum of three populations.
Since the response variable is binary (1 for positive and 0 for negative), data were analyzed using logistic regression. The regression was ran in R using the function glm() with binomial(link = “logit”). Within the framework of the logistic regression model, contrasts between population, gender, and serotype were calculated using “emmeans” package (version 1.6.2-1).
In addition, comparison were conducted using Fisher Exact Test [fisher.test() function]. For all contrasts and comparisons, odd ratios and nominal (not adjusted for multiplicity) p-values were reported. Pearson correlation between NAb and BAb was calculated using log transformed values. For NAb titer, <5 was imputed as 2.5 and >40 was imputed as 80. Additional statistical analysis was performed using GraphPad Prism.
Results
We evaluated serum from two sets of donors; cohort 1 comprised 100 donors, while cohort 2 had 300 donors. All donors were healthy and self-identified as belonging to one of the three major race groups within the United States. The breakdown of cohort 1 and 2 by race, gender, and age is shown in Table 1.
Table 1.
Donor characteristics by race, gender, and age
| |
|
|
|
Age |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race |
Total |
Male |
Female |
20–30 |
31–40 |
41–50 |
51–60 |
61–70 |
71–80 |
||||||
| M | F | M | F | M | F | M | F | M | F | M | F | ||||
| Cohort 1 | |||||||||||||||
| White | 32 | 16 | 16 | 0 | 1 | 1 | 2 | 5 | 3 | 6 | 6 | 3 | 4 | 1 | — |
| Hispanic | 34 | 17 | 17 | 1 | 3 | 3 | 3 | 5 | 6 | 6 | 3 | 2 | 2 | — | — |
| Black | 34 | 17 | 17 | 4 | 4 | 1 | 8 | 2 | 2 | 5 | 3 | 4 | — | 1 | — |
| Cohort 2 | |||||||||||||||
| White | 100 | 50 | 50 | 5 | 10 | 3 | 17 | 7 | 13 | 30 | 8 | 5 | 2 | — | — |
| Hispanic | 100 | 50 | 50 | 13 | 15 | 6 | 12 | 10 | 13 | 12 | 9 | 9 | 1 | — | — |
| Black | 100 | 50 | 50 | 13 | 12 | 11 | 9 | 12 | 12 | 11 | 14 | 3 | 3 | — | — |
Self-identified donors belonging to different races, gender, and age.
M, male; F, female.
AAV NAb seroprevalence
A cell-based transduction inhibition assay was used to evaluate presence (titer ≥5) or absence (titer <5) of NAbs in cohort 1 subjects against a panel of vectors (AAV1, AAV3B, AAV5, AAV9, AAVrh74, Spark100, and AAVDJ). Since our data set did not accurately represent the available census data of the United States, the data were demographically corrected (weighted) to arrive at the overall estimated NAb seroprevalence in the population. The demographic correction was based on the published/reported population estimates of white 60.1%, black 13.4%, Hispanic or Latino 18.5%, and other races 8% within the United States (U.S. census bureau July 1, 2019; https://www.census.gov/quickfacts/fact/table/US/PST045219).
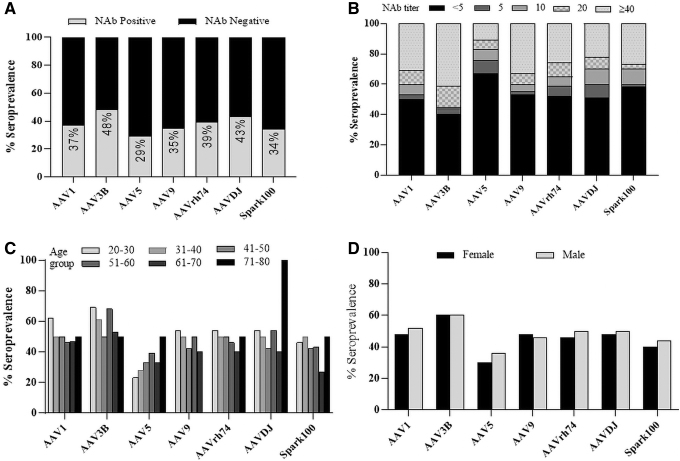
The overall weighted seroprevalence of all vectors was below 50% (Fig. 1A). Of these, AAV1, AAV9, AAVrh74, and Spark100 seroprevalence was more similar (37%, 35%, 39%, and 34%, p > 0.05 in all pairwise comparisons), while AAV5 was lower at 29%. AAVDJ and AAV3B seroprevalence was marginally higher at 43% and 48%, respectively. The only significant difference among capsids was the lower incidence of AAV5 NAbs when compared to AAV3B and AAVDJ (p = 0.007 and 0.047, respectively).
Figure 1.
AAV seroprevalence and titer distribution among healthy donors in the United States. A NAb assay was used to evaluate seroprevalence and titers in 100 healthy donors within the United States against a panel of AAVs. (A) Adjusted seroprevalence among donors to multiple AAVs. Data are shown as seroprevalence percentage of NAb positive (grey; NAb titer ≥5) and negative (black; NAb titer <5) donors. (B) Titer distribution among all donors (unadjusted) against AAVs. (C) Seropositivity among all age groups. Donors were binned by age before calculating percent seropositivity within each age group. (D) Gender distribution of seropositive donors against AAVs. AAVs, adeno-associated viral vectors.
Although we had equal distribution of donors, the weighted seroprevalence assigned lower weights to black and Hispanic donors than white donors. To investigate differences between donors more accurately, we analyzed the unweighted data that included all 100 donors. Subjects were first stratified based on their NAb titers (<5, 5, 10, 20, and ≥40) against each vector (Fig. 1B). Among the unweighted NAb-positive donors, more had high-titer antibodies (titers >40) to all AAVs except AAV5. For instance, 40% of donors had a NAb titer >40 against AAV3B in contrast to 10% against AAV5. Seroprevalence of high-titer antibodies against other capsids ranged between 25% and 35%. Differences among subjects with low level NAb (titers 5–20) to other capsids was less remarkable.
We next analyzed the seroprevalence by age. Donors were binned into different age groups based on the decade of life and seroprevalence was estimated within each bin (Fig. 1C). AAV5 seroprevalence was lower in younger subjects aged younger than 30 years (22%) compared to other vectors (45–70%). Seroprevalence of other AAVs did not vary remarkably by age except for AAV5. Although a higher incidence of AAVDJ was observed in subjects >70 years, the number of subjects in this age group is limited (n = 2) and did not allow for any meaningful conclusion.
There were no differences in overall seroprevalence between men and women (Fig. 1D). However, when we stratified donors by NAb titers, we did observe twice as many men had high-titer NAbs to AAV5 (titer >40) compared to women (11% vs. 6%, men vs. women, Supplementary Fig. S1). Except for AAV5, we did not observe differences between men and women against any other capsids.
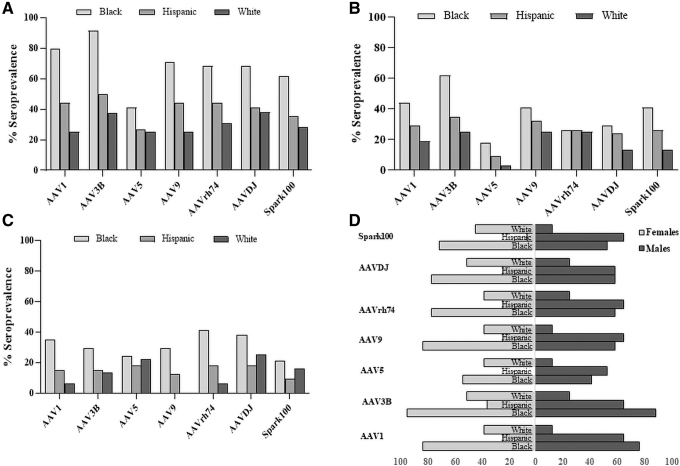
Higher seroprevalence among minorities
Unweighted seroprevalence deviated most among races with 79% of black donors positive for AAV1 NAbs (Fig. 2A). In contrast, only 25% of whites were AAV1 positive. Although Hispanic AAV1 seroprevalence was higher (44%), it was not significantly different from white donors (p = 0.107). Similarly, AAV3B seroprevalence was higher among blacks (>90%) and lower in Hispanics and white donors (50% and 38%, respectively). AAV9, AAVDJ, and Spark100 seroprevalence was also skewed with more black donors seropositive compared to whites and Hispanics (p < 0.05).
Figure 2.
AAV seroprevalence and titer distribution among different races. (A) AAV seroprevalence among different races. Data are shown as seropositivity among all donors who self-identified as black, Hispanic, or white. (B) Distribution of donors with high-titer NAbs (>40) by race. (C) Distribution of donors with low-titer NAbs (5 to ≥20) by race. (D) Gender distribution of seropositive donors by race. Data are plotted as seropositive males (dark bars) or females (white bars) within each race. Numbers on the bottom indicate percent seroprevalence.
For AAV5, when compared with whites and Hispanic, black donors had numerically higher seroprevalence, however, the differences were not significant (p = 0.066 and 0.0815, respectively). These racially skewed NAb seroprevalences were more evident in donors with high-titer (>40) antibodies to AAV1, AAV3B and Spark100 (p < 0.05; Fig. 2B). Among these donors, seroprevalence was higher in blacks when compared to whites, and no difference was found between other races. Only AAV1 and AAVrh74 seroprevalence was significantly higher among black donors with low NAb titer when compared to whites (Fig. 2C; p = 0.0098 and 0.037, respectively).
NAb seroprevalence by gender
First, we compared intraracial differences in AAV seroprevalence. Overall, Hispanic men were more seropositive compared to women (Fig. 2D). For instance, Spark100 seroprevalence among Hispanic men and women was 65% and 6%, respectively. Similarly, AAV5 seroprevalence was 53% among Hispanic males, while no Hispanic women was AAV5 seropositive. Among other capsids, Hispanic men were twice as likely to be AAV NAb positive. The increased NAb incidence among Hispanic men compared to women was not observed in other races. AAV seroprevalence among white women did not differ significantly from their male counterparts, although there appeared to be a trend toward higher seroprevalence in women. Similarly, no notable differences were observed among black women and men.
Next, we compared interracial differences by gender. Among men, seroprevalence between blacks and Hispanics was similar (p > 0.05) and both were more likely to be positive compared to whites. Blacks had significantly higher (p < 0.05) seroprevalence to AAV1, AAV3B, AAV9, and Spark100 when compared to whites. Similarly, Hispanic seroprevalence was significantly higher (p < 0.05) against all AAVs compared to whites except for AAVDJ. However, among women, blacks had higher seroprevalence to all AAVs compared to Hispanics (p < 0.05). Interestingly, seroprevalence was more similar between black and white women (p > 0.05) for AAV1, AAV3B, and AAV9.
Cross-reactive NAbs
We next investigated cross-reactivity against different capsid in these donors. Our choice of vectors belonged to multiple clades; for instance, AAV1, AAVrh74, and AAV9 belong to clade A, E, and F, respectively. However, both AAV3B and AAV5 do not belong to any known clades. While AAV3B is closer to viruses in clade A or C, AAV5 is classified into a separate clade. Both the bioengineered Spark100 and AAVDJ, an engineered capsid that was isolated following capsid shuffling, are yet to be classified into a clade. Despite these differences, there was considerable cross-reactivity among different AAVs in our study. As an example, all subjects who had NAbs to AAV1 also cross-neutralized AAV3B (Table 2). While cross-reactivity to Spark100 and AAVDJ was marginally lower (82% and 88%, respectively), it was the lowest against AAV5 (60%).
Table 2.
Cross-neutralization of capsids by donor sera
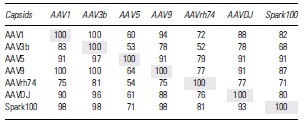
|
Analysis of donor sera when positive against an AAV (row) to cross-neutralize other serotypes (columns). Numbers represent percent seropositivity among all NAb-positive donors.
AAV, adeno-associated viral vector; NAb, neutralizing antibody.
Interestingly, not all subjects who had AAV3B NAbs cross neutralized AAV1, although the levels were still high at 83%. Similar findings were also noted for all tested capsids with the following observations: (1) >90% subjects who were AAV5 positive also cross-neutralized other serotypes except for AAVrh74 (79%). (2) However, subjects NAb positive to other capsids only weakly neutralized AAV5. (3) Nearly all subjects with NAbs to Spark100 also cross-neutralized AAV1, AAV3B, AAV9, and AAVDJ (98%, 98%, 98%, and 93%, respectively) with the exception of AAVrh74 (79%). As an example, only 53% of AAV3B NAb-positive donors cross-neutralized AAV5. The highest AAV5 cross-neutralization was observed with Spark100 NAbs (71%).
We next stratified and analyzed NAb cross-reactivity in donors who had high or low NAb titers against an AAV (Supplementary Fig. S3, top left and right panels, respectively). Donors with high-titer NAb to a capsid also cross-neutralized other serotypes. However, donors with low-titer NAbs to a capsid were less likely to neutralize other serotypes. Donors with high-titer NAbs to an AAV also had high-titer NAbs to a heterologous serotype (Supplementary Fig. S3, bottom left panel). Conversely, most donors with low-titer NAbs to capsid were less likely to carry higher titer antibodies to a heterologous capsid (Supplementary Fig. S3, bottom right panel).
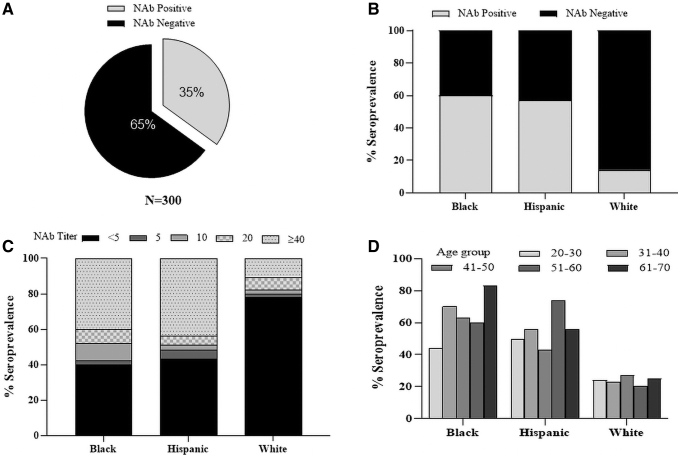
NAb seroprevalence in cohort 2
Our analysis of cohort 1 donors demonstrated that seroprevalence against several capsids was unexpectedly higher among blacks and Hispanic donors. To confirm these findings, we obtained serum from 300 additional donors comprised of equal numbers of black, Hispanic, and whites (Table 1). Serum from all donors was evaluated for presence of AAV1 NAbs. The weighted AAV1 seroprevalence was 35% and was similar to our observations in cohort 1 donors (Fig. 3A).
Figure 3.
AAV1 seroprevalence in a second cohort of 300 donors. (A) Demographically adjusted AAV1 seroprevalence. (B) Seropositivity among all donors who self-identified as black, Hispanic, or white. (C) Seropositive donors were stratified based on NAb titer with each race. (D) Donors were binned by age before calculating seropositivity by race.
The unweighted analysis also demonstrated nearly four-fold higher NAb seroprevalence among black and Hispanic donors when compared to whites (60% and 57% vs. 14%, Fig. 3B). Furthermore, both black and Hispanic donors also carried high-titer antibodies (Fig. 3C). We did not observe a skewed NAb seroprevalence by age among the races, although there appeared to be a trend toward marginally higher seropositivity among older blacks (Fig. 3D). We did not observe any gender differences in seroprevalence.
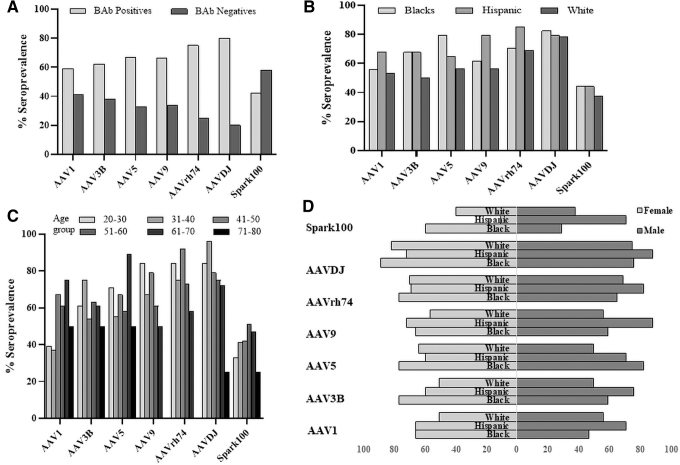
IgG BAbs
The skewed NAb seroprevalence among minority races motivated us to investigate capsid IgG BAbs in these donors. We evaluated BAbs in all cohort 1 donors using an ELISA-based assay (Fig. 4A). Except for AAV9, AAVrh74, and AAVDJ, there was good correlation between NAbs and BAbs (correlation coefficient >0.58; Supplementary Fig. S2). We did observe a bias in higher BAbs seroprevalence among minority donors against only AAV3B and AAV5 capsids. In general, the BAbs seroprevalence was less skewed toward minority donors compared to NAbs (Fig. 4B). Interestingly, there was a trend toward higher BAb seroprevalence against AAV1 and Spark100 in donors aged 40 to 70 years that contrasted with the lack of any age bias in NAb seroprevalence among most capsids (Fig. 4C). There was no difference in BAbs seroprevalence between the genders (Fig. 4D) and was consistent with our observations comparing NAb seroprevalence between men and women.
Figure 4.
Seroprevalence of BAbs. (A) AAV IgG seroprevalence in demographically adjusted donors. (B) Seroprevalence among donors who self-identified as belonging to one of three races. (C) Donors were binned by age before calculating seropositivity by race. (D) Distribution of seropositive donors by gender. BAbs, binding antibodies.
Discussion
Systemic AAV gene therapy has been demonstrated to be ineffectual in animal models and patients with preexisting NAb.5,10 The studies presented here were actuated to understand differences in AAV NAb seroprevalence enabling capsid selection during AAV drug development. Higher seroprevalence results in deselection of a capsid that would otherwise have all desired traits. As part of a pilot screening process, we noted considerable variability in AAV seroprevalence among donors (data not shown). To better understand the source of these variations, we undertook the present study evaluating NAb seroprevalence among donors based on race, gender, and age against a panel of AAV capsids. The selection of capsids was sufficiently diverse and comprised vectors belonging to multiple clades with a propensity for targeting liver following systemic administration.11
Of these, AAV1 and AAVrh74 were isolated from primates while AAV3B, AAV5, and AAV9 were naturally occurring isolates from humans.11–14 Among other vectors we investigated, Spark100 was a bioengineered vector and AAVDJ was a novel variant isolated from a capsid library following DNA shuffling and expected to be less neutralized by antibodies.15,16 The diversity of capsids allowed us to interrogate seroprevalence differences against both natural isolates and engineered capsids.
Our findings demonstrate a lower seroprevalence (<50%) against most capsids. The overall adjusted seroprevalences were consistent with previous published reports with these capsids with some differences.8,17,18 The greatest variability was observed in AAV5 seroprevalence. It is likely that the assays to interrogate the presence of AAV5 NAbs require optimization before observing alignment between studies. As a note, a clinical trial for AAV5.hFIX recruited and dosed five NAb-negative patients with hemophilia B.19 Development of a more sensitive assay later found that three out of the five patients had preexisting NAbs. AAV5 is known to be structurally and antigenically quite distinct from other AAVs, and this may contribute to the observed discrepancy between studies evaluating its seroprevalence.20
AAV antibody seroprevalence can be evaluated using either the NAb or BAb assay.21 The NAb assay is a functional assay measuring transduction inhibition as a surrogate for presence of anti-AAV NAbs. In contrast, the ELISA-based BAb assay measures total IgG that bind vector. In our studies, more subjects were BAb positive than NAb positive. As an example, twice as many donors had AAV5 BAbs compared to NAbs (60% vs. 30%, respectively). Higher incidence of BAb among NAb-negative subjects has previously been reported in a screen of anti-AAV antibodies.18
It is conceivable that NAbs represent high-affinity antibodies generated after one or more AAV exposures, while BAbs include both high- and low-affinity antibodies. Our studies demonstrate cross-reactivity against AAVs belonging to distinct clades and donors with high-titer antibodies mostly cross-neutralized multiple AAVs. Presence of high-titer cross-reactive antibodies may allude to donors being infected with multiple AAVs, or generation of high-affinity broadly NAbs against shared epitopes.
The most interesting observation from our studies was the higher NAb seroprevalence among black and Hispanic donors to most AAVs. These interracial differences were evident in male donors, while differences among women were lower. Intraracial differences were higher between the genders with white women and Hispanic men more seropositive compared to their counterparts. The disproportionately higher NAb incidence among minority donors motivated us to confirm the findings in a second larger set of samples. Serum from 300 additional donors in cohort 2 confirmed our earlier findings of higher seroprevalence among minorities.
Similarly, a higher incidence of AAV2 and AAV8 NAbs was also observed among minority donors in two different donor cohorts from the same geographical location. While seroprevalence was higher among black and Hispanic donors to AAV8, only black donors had a higher seroprevalence to AAV2 (data not shown). The skewed seroprevalence between races did not affect the overall estimated population seroprevalence of the weighted sample set. The skewed NAb seroprevalence was also observed with BAbs, although it was less pronounced.
Few reports have evaluated differences in AAV NAb prevalence among donors based on race. Our findings are in variance with a published report on the lack of differences in AAV NAb seroprevalence among races.22 In the study by Ellsworth et al., NAbs were evaluated against Clade F AAVs in 100 donors within the United States and the authors did not observe any statistically significant difference based on race. There are important differences between our studies: First, the vectors, cells, and assays used for evaluating NAb titers differed in the two studies. Second, and more importantly, except for 12 donors in cohort 1, all donors in our study were from a single geographical location (Florida, U.S.). It was not specified whether samples in the Ellsworth study were obtained from the same or different geographical locations.
The ongoing coronavirus disease 2019 (COVID-19) pandemic has prevented us from sampling donors at different locations within the United States. It is conceivable that the higher NAb seroprevalence among minority races may be limited to this single location within the United States. It is unclear why AAV seroprevalence was higher among minorities and in some races, especially males? Increased NAb incidence among some races may correlate with higher multigenerational households. Most of our donors were from Florida; a state with a higher percentage of multigenerational households (/www.census.gov/content/dam/Census/library/working-papers/2013/acs/lofquist-01.pdf). The authors acknowledge that other socioeconomic factors may have also contributed to these differences.
To our knowledge, this is the first report on a disproportionate increase in NAb incidence among minority races in a geographical location. The findings here underscore the impact of sampling error in estimating population seroprevalence. We suggest that capsid selection for drug development take into consideration AAV seroprevalence among the patient population while avoiding sampling bias.
Supplementary Material
Acknowledgments
The authors thank the Viral Vector Core Facility at UMASS for providing AAV1, AAV3B, AAV5, AAV9, AAVDJ, and AAVrh74. We thank Dr. Seng H. Cheng, Dr. Pan Clark, and Dr. John E. Murphy for useful discussions.
Authors' Contributions
A.K. designed the study, sourced the material, conducted the studies, interpreted the data, prepared the figures, and cowrote parts of the article. R.S. produced the Spark100 vector used in the studies. S.G. performed the statistical analysis and cowrote parts of the article. S.S. conceived the study, coordinated research strategy, and wrote the article. All coauthors have read and agreed with the contents of the article.
Author Disclosure
All authors are employees of Pfizer, Inc. and have no conflicts of interest to declare.
Funding Information
This study was supported by Pfizer.
Supplementary Material
References
- 1. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 2019;18:358–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuzmin DA, Shutova MV, Johnston NR, et al. . The clinical landscape for AAV gene therapies. Nat Rev Drug Discov 2021;20:173–174. [DOI] [PubMed] [Google Scholar]
- 3. Naso MF, Tomkowicz B, Perry WL, 3rd, et al. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017;31:317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Calcedo R, Bell P, et al. . Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347. [DOI] [PubMed] [Google Scholar]
- 6. Ronzitti G, Gross DA, Mingozzi F. Human immune responses to adeno-associated virus (AAV) vectors. Front Immunol 2020;11:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Somanathan S, Calcedo R, Wilson JM. Adenovirus-antibody complexes contributed to lethal systemic inflammation in a gene therapy trial. Mol Ther 2020;28:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perocheau DP, Cunningham S, Lee J, et al. . Age-related seroprevalence of antibodies against AAV-LK03 in a UK population cohort. Hum Gene Ther 2019;30:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ling C, Wang Y, Feng YL, et al. . Prevalence of neutralizing antibodies against liver-tropic adeno-associated virus serotype vectors in 100 healthy Chinese and its potential relation to body constitutions. J Integr Med 2015;13:341–346. [DOI] [PubMed] [Google Scholar]
- 10. Rapti K, Louis-Jeune V, Kohlbrenner E, et al. . Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther 2012;20:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao G, Vandenberghe LH, Alvira MR, et al. . Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao W, Chirmule N, Berta SC, et al. . Gene therapy vectors based on adeno-associated virus type 1. J Virol 1999;73:3994–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol 1998;72:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiorini JA, Kim F, Yang L, et al. . Cloning and characterization of adeno-associated virus type 5. J Virol 1999;73:1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George LA, Sullivan SK, Giermasz A, et al. . Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017;377:2215–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimm D, Lee JS, Wang L, et al. . In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol 2008;82:5887–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712. [DOI] [PubMed] [Google Scholar]
- 19. Majowicz A, Nijmeijer B, Lampen MH, et al. . Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing anti-AAV5 NABs. Mol Ther Methods Clin Dev 2019;14:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Govindasamy L, DiMattia MA, Gurda BL, et al. . Structural insights into adeno-associated virus serotype 5. J Virol 2013;87:11187–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anguela XM, High KA. Oracle or false prophet? Can we predict AAV efficacy based on preexisting antibody titers? Res Pract Thromb Haemost 2019;3:149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellsworth JL, O'Callaghan M, Rubin H, et al. . Low seroprevalence of neutralizing antibodies targeting two clade F AAV in humans. Hum Gene Ther Clin Dev 2018;29:60–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.