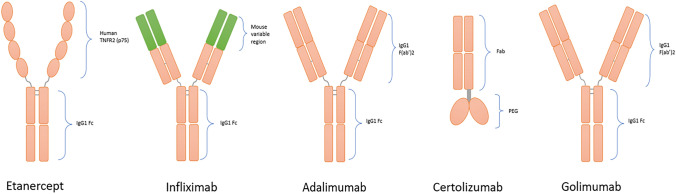
Fig. 1.
Molecular structure of approved anti-TNFs. The five approved anti-TNFs, presented in chronological order of first approval. Etanercept is a fusion protein of extracellular domain (p75) of human TNFR2 and Fc fragment of IgG1; infliximab is a mouse/human chimeric monoclonal IgG1 anti-TNF-α antibody; adalimumab is a humanized IgG1 monoclonal anti-TNF-α antibody; certolizumab is a Fc-free Fab region of a recombinant humanized IgG1 monoclonal anti-TNF-α antibody, conjugated to PEG; golimumab is a human IgG1 monoclonal anti-TNF-α antibody. Anti-TNF-a: tumor necrosis factor alpha inhibitor, PEG: polyethylene glycol, TNFR2: tumor necrosis factor receptor 2