Fig. 3.
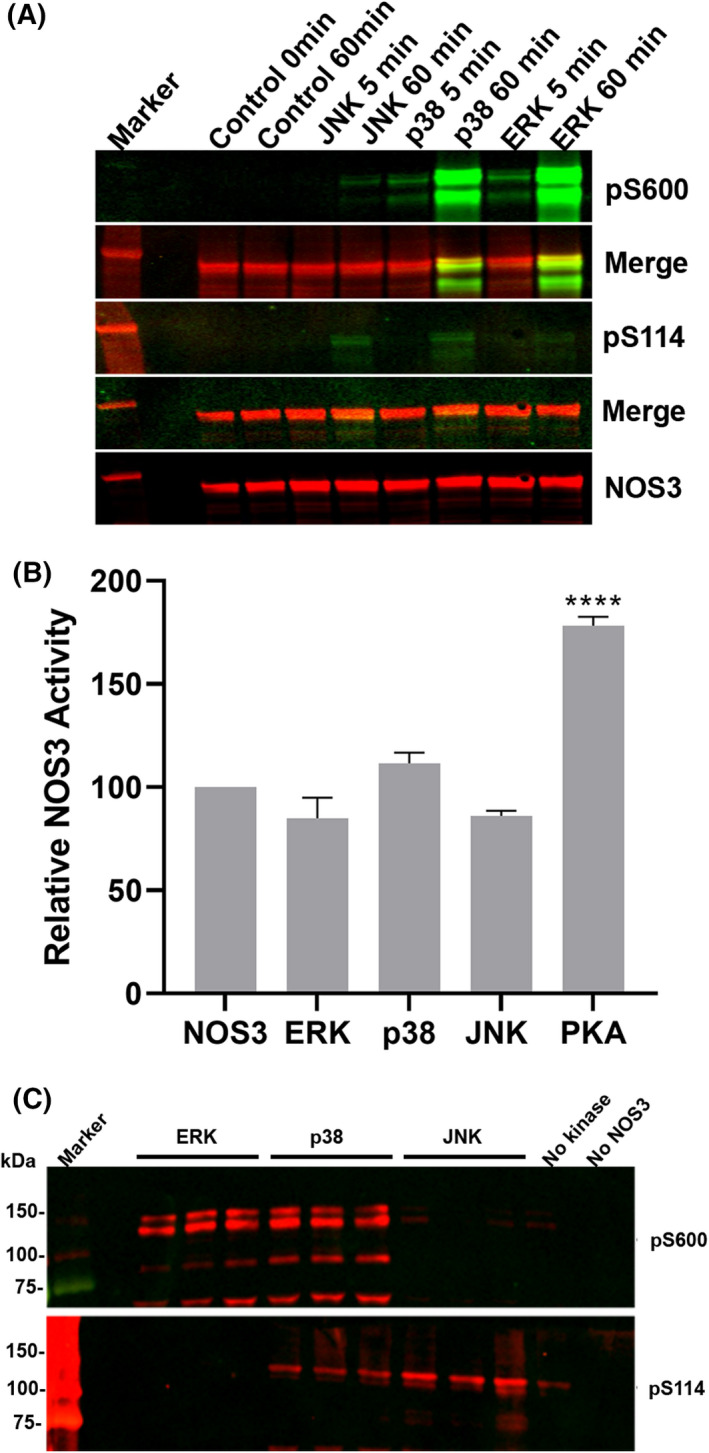
JNK1α1, p38α, and ERK2 phosphorylate NOS3 at different sites but do not significantly change NO production. (A) Active kinases, JNK1α1 (0.16 µg), p38α (0.68 µg), or ERK2 (0.16 µg), were incubated in kinase reactions with NOS3 (150 µg), samples were taken at 5 and 60 min and analyzed by immunoblot using phospho‐specific antibodies S600 [6] and S114 green (Millipore) and anti‐NOS3 red (BD Biosciences), yellow indicates both signals. (B) NOS3 (2.5 µm) was incubated with either ERK2 (14 nm) N = 10, p38α (14 nm) N = 4, JNK1α1‐GST (63 nm) N = 3, or PKA (14 nm) N = 4, for 30 min in kinase reactions were used to measure the change in oxyhemoglobin (401 nm) to methemoglobin (421 nm) in triplicate for 30 min. Approximately 8 µg of NOS3 from the kinase reaction was used in each of the OxyHb. One‐way ANOVA test was used to calculate the P values (****P < 0.001). The data are expressed as mean ± SD. (C) Immunoblot analysis of NOS3 from one of the OxyHb assays in triplicate.
