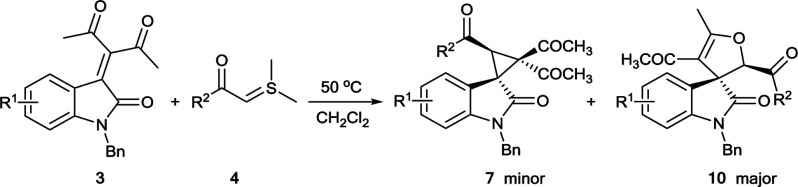
Synthesis of dihydrofuran-fused spirooxindolea.
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Yieldb (%) (7/10) | d.r.c |
| 1d | H | Ph | 15/74 | 10 : 1 (10a) |
| 2 | H | 2-FC6H4 | 13/66 | 16 : 1 (10b) |
| 3 | H | 4-FC6H4 | 11/68 | 16 : 1 (10c) |
| 4 | H | 3,4-Cl2C6H3 | 12/71 | 8 : 1 (10d) |
| 5 | H | 4-BrC6H4 | 10/75 | 10 : 1 (10e) |
| 6 | H | 2-CH3C6H4 | 8/78 | 20 : 1 (10f) |
| 7 | H | 4-OCH3C6H4 | Trace/81 | 18 : 1 (10g) |
| 8 | H | Thienyl | 15/60 | 5 : 1 (10h) |
| 9 | H | Naphthyl | 15/62 | 5 : 1 (10i) |
| 10 | H | CH3 | 14/74 | 6 : 1 (10j) |
| 11 | H | OEt | 13/76 | 5 : 1 (10k) |
| 12 | 5-F | Ph | 8/70 | 10 : 1 (10l) |
| 13 | 7-F | Ph | Trace/56 | 16 : 1 (10m) |
| 14 | 5-Cl | Ph | 14/66 | 5 : 1 (10n) |
| 15 | 6-Cl | Ph | 12/67 | 15 : 1 (10o) |
| 16 | 5-Br | Ph | 10/70 | 13 : 1 (10p) |
| 17 | 6-Br | Ph | 10/69 | 10 : 1 (10q) |
| 18 | 5-CH3 | Ph | 8/80 | 18 : 1 (10r) |
| 19e | H | Ph | 12/80 | 9 : 1 (10a) |
Unless otherwise noted, all reactions were performed with 3 (0.15 mmol), 4 (0.165 mmol) in 2 mL DCM at 50 °C for 4 h.
Isolated yields of the compound 7 and 10.
The diastereoselective ratio of compounds 10 were determined by 1H-NMR analysis of the crude reaction mixture.
The relative configuration of 10a was determined by X-ray crystallographic analysis (Fig. 2), and the relative configurations of other products 10 were tentatively assigned by analogy.
A gram scale reaction of 3a (3.13 mmol) and 4a (3.44 mmol) in DCM at 50 °C was carried out.
