Correction to: 10.1007/s40120-022-00341-z
In the published article a few errors have been identified, these have subsequently been rectified. Alterations were also made to sentences and reference numbering to improve the clarity of sentences and better reflect the published literature. These changes did not impact upon the conclusions of the article.
The specific corrections made are provided below:
Abstract
The value of 14.3% has been amended to 14.4% as the calculated value was 14.38% ~ 14.4%.
Introduction:
The value 9.7% was replaced with 15.5%, and the value 1.6% was replaced with 1.97% to align with the value from cited reference number 9 (Prosperini L, et al. 2021).
Results:
The value 69.6% was replaced with accurate value 69.8%.
Discussion:
Value 1.6% was altered to 1.4% which is reported as the minimum value for mortality in multiple sclerosis (MS) patients treated with disease modifying therapies (DMTs). This value refers to mortality rate for natalizumab-treated MS patients. This aligns with data taken from reference number 12 (Simpson-Yap S, et al.).
Value 21.5% was replaced with 20.9% as a maximum range value for hospitalization rate specific to general MS population. This aligns with data taken from reference number 12. (Simpson-Yap S, et al.).
The value 13.3% was changed to 11.5% as a minimum range value from the cited references for hospitalization rate in MS patients treated with DMTs. This value refers to hospitalization rate for natalizumab-treated MS patients. This aligns with data taken from reference number 12. (Simpson-Yap S, et al.).
The statement “Earlier studies found a higher risk of serious infections in patients with low IgG/IgM levels taking ocrelizumab” has been modified to “Earlier studies found an increased risk of serious infections in patients with low IgG levels taking ocrelizumab”, taken from the newly added reference number 36 Hauser SL, et al.
Figure 1:
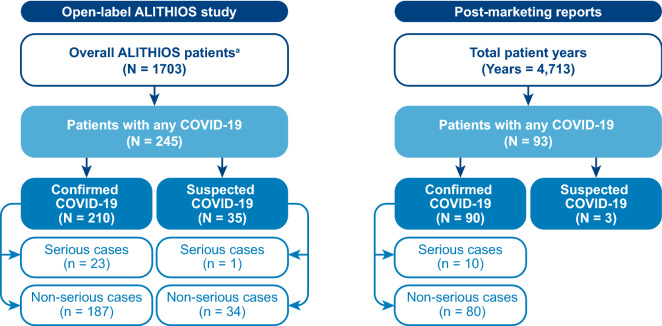
- The number of serious cases and non-serious cases in the post-marketing reports section were corrected to Serious cases (n = 10) and Non-serious cases (n = 80).
Fig. 1.
Patient designation in the open-label ALITHIOS study and post-marketing setting. a Patients in the open-label ALITHIOS study (N = 1703) rolled-over from the APOLITOS (n = 57), APLIOS (n = 279), and ASCLEPIOS I/II (n = 1367) studies
Figure 2:
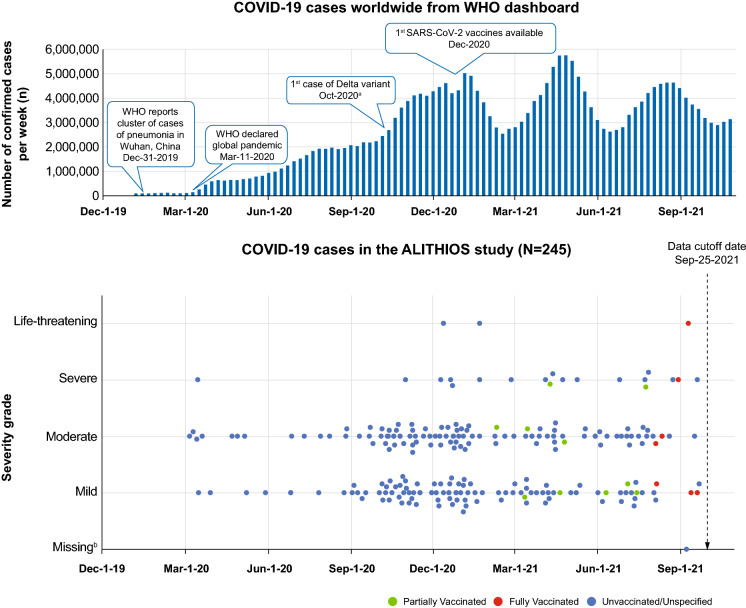
- The date listed for when the WHO declared a global pandemic was incorrect. This has been updated MARCH 11, 2020.
Fig. 2.
COVID-19 cases over time in the open-label ALITHIOS study by severity and vaccine status. aFirst case of Omicron variant reported after data cut-off: November 24, 2021. bSeverity grade for one patient was not yet reported at the time of data cut-off, as ALITHIOS is an ongoing study, and the data are subject to change. COVID-19, coronavirus disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WHO, World Health Organization
References
Reference 36 replaced with new reference: Hauser SL, Kappos L, Montalban X, et al. Safety of Ocrelizumab in Patients With Relapsing and Primary Progressive Multiple Sclerosis Neurology. 2021 Oct 19; 97(16): e1546–e1559. 10.1212/WNL.0000000000012700.
Reference 37 replaced with former #36: Jasin´ska E, Habek M, Wynn D, Dunphy S, Mancione L, Rennie N, Su W, Zielman R, Delgado S. Impact of ofatumumab on immune responses post-vaccination in RMS patients: ALITHIOS vaccination substudy design. In: Presented at 7th Congress of the European Academy of Neurology, (Virtual); June 19–22, 2021, OPR-207.
Reference 42 replaced with new reference: Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. 10.1038/s41591-021-01507-2.
The original article has been corrected.