Abstract
Background Context:
A variety of solutions have been suggested as candidates for the repair of the annulus fibrosis (AF), with the ability to withstand physiological loads of paramount importance.
Purpose:
The objective of our study was to capture the scope of biomechanical test models of AF repairs. We hypothesized that common test parameters would emerge.
Study design:
Systematic Review
Methods:
PubMed® and EMBASE databases were searched for studies in English including the keywords “disc repair AND animal models”, “disc repair AND cadaver spines”, “intervertebral disc AND biomechanics”, “disc repair AND biomechanics”. This list was further limited to those studies which included biomechanical results from annular repair in animal or human spinal segments from the cervical, thoracic, lumbar and/or coccygeal (tail) segments. For each study, the method used to measure the biomechanical property and biomechanical test results were documented.
Results:
A total of 2,607 articles were included within our initial analysis. Twenty-two articles met our inclusion criteria. Significant variability in terms of species tested, measurements used to quantify annular repair strength, and the method/direction/magnitude that forces were applied to a repaired annulus were found. Bovine intervertebral disc was most commonly used model (6/22 studies) and the most common mechanical property reported was the force required for failure of the disc repair device (15 tests).
Conclusions:
Our hypothesis was rejected; no common features were identified across AF biomechanical models and as a result it was not possible to compare results of pre-clinical testing of annular repair devices. Our analysis suggests that a standardized biomechanical model that can be repeatably executed across multiple laboratories is required for the mechanical screening of candidates for AF repair.
Keywords: Annular repair, annular repair devices, biomechanical testing, pre-clinical models, disc repair, animal studies, testing methodology, failure load, spine model, discectomy repair, hydrogel, annular closure
Introduction
Disc herniations can cause focal low back pain and debilitating lumbar radicular symptoms[1, 2]. Low back pain is the top cause for years lived with disability in the United States in 1990–2016[3]. Socio-economic costs for the treatment of low back pain of up to $90 billion have been estimated [4–11]. A proven treatment for symptomatic disc herniation is a discectomy procedure where a portion of a herniated disc is removed allowing for decompression of the thecal sac and exiting nerve roots[12]. 0.5–7.9% of surgeries require revision discectomy due to reherniation[13–16], suggesting continued degeneration of an intervertebral disc/endplate even after a discectomy[17–20]. It has been hypothesized that repairing the annulus fibrosus (AF) might halt the pathophysiology behind disc reherniation after a microdiscectomy[21, 22], but there has yet to be a single device with long term data supporting widespread clinical use[23].
One of the barriers to the widespread use of a disc repair devices is clinical concern over the consequences of displacement of a device within the delicate anatomical space around an intervertebral disc. While numerous devices have been invented to repair defects in the annulus fibrosus, no study has compared results across multiple test systems. This deficiency is particularly important because intervertebral discs plays a vital role in maintaining function and allowing for flexibility within the lumbar spine[24, 25]. The AF experiences loads including tensile, compressive and shear stresses/strains[26, 27]. The lumbar spine, in particular, is at risk for degenerative changes due to the fact that it has a large range of motion and the lordotic alignment of disc spaces places more pressure over the posterior annulus fibrosus during range of motion[28–30]. Further disruption during the lumbar discectomy procedure accelerates intervertebral disc degeneration and may play a role in discogenic back pain[28, 31–33].
The objective of our study was to capture the scope of all biomechanical test models as applied to the intervertebral disc. Our hypothesis was that common test parameters would emerge.
Methods
Study Selection:
The Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) methodology was used to prepare our manuscript[34, 35]. Only articles written in English were considered. The PubMed® and EMBASE databases were queried using the search terms “Intervertebral disc AND disc repair AND biomechanics”, “Disc repair AND animal model”, “Disc repair AND human cadaver spine”, “Annulus closure AND spine” or “Intervertebral disc AND disc repair”. A total of 2,607 studies were identified between 1990 to 2018. No abstracts/proceedings from scientific conferences were analyzed within our search process due to insufficient detail within these studies to accurately describe/perform their mechanical testing protocol. We further refined our search to include publications that created mechanically induced disc herniations, and studies that quantified the biomechanical properties of spines treated with a disc repair device. Studies that dealt with a nucleus replacement were not included within this assessment as we were focused on disc repair devices[36–38]. Articles that included only a nucleus replacement without an annular repair were not included in the review.
Analysis of Studies:
Peer reviewed published papers were categorized based on the following criteria: (i) mode of control (ii) dynamic vs. cyclic (iii) apparatus (iv) region of spine (v) species (vi) type of repair and use of a control (vii) primary outcome metric. A master-table was created to capture the scope across the defined categorizations. We paid particular attention to models that were iterated within/across groups. Finally, the limitations of the models in the clinical context of known failure modes and the intended use of candidate materials for AF repair was discussed. The authors have no current direct relationships with companies that produce annular repair devices nor do we have any intellectual property related to annular repair devices.
Results
Articles identified:
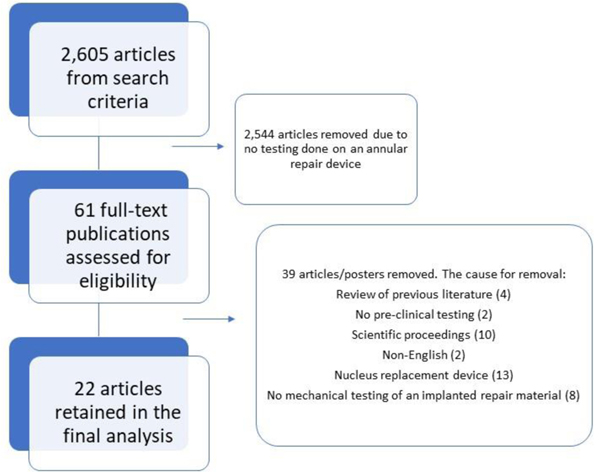
Our search terms yielded 2,607 articles. Using our inclusion criteria, 22 articles were identified that had mechanical testing results from an intervertebral annular repair material[21, 22, 28, 39–66]. An overview of our search results is shown in Figure 1. These articles are summarized in Table 1. A breakdown of key conclusions from each article is shown in Table 2.
Figure 1:
The methodology used for our systematic review, resulted in an analysis of 22 papers.
Table 1:
Summary table showing the articles reviewed and highlighting key characteristics of each experiment. Note the wide variety of species tested and primary outcomes from the studies.
| Mode of Control | Cyclic vs static | Apparatus | Region of spine | Primary outcome | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citation | Species | Controltested? | Barrierimplant? | Hydrogel tested? | Axialload/Pressureload | Axialdisplacement | Torque | Torsion angle | Flexion torque | Flexion angle | Dynamic testing | Static testing | Custom apparatus? | Standard apparatus? | Cervical | Thoracic | Lumbar | Tail | Failure | ROM | Stiffness | Displacement | Hydraulic permeability | Adhesion strength | Intradiscal pressure |
|
| |||||||||||||||||||||||||
| Ahlgren, 2000 [22] | Sheep | x | x | x | x | x | x | x | |||||||||||||||||
|
| |||||||||||||||||||||||||
| Bateman, 2016 [40] | Porcine | x | x | x | x | x | x | x | x | ||||||||||||||||
|
| |||||||||||||||||||||||||
| Borde, 2015 [41] | Rat tail | x | x | x | x | x | x | x | x | ||||||||||||||||
|
| |||||||||||||||||||||||||
| Bostelman, 2017 [66] | Human | x | x | x | x | x | x | x | x | x | x | ||||||||||||||
|
| |||||||||||||||||||||||||
| Bron, 2010 [42] | Goat | x | x | x | x | x | x | x | x | x | |||||||||||||||
|
| |||||||||||||||||||||||||
| Chiang, 2011 [44] | Porcine | x | x | x | x | x | x | x | x | ||||||||||||||||
|
| |||||||||||||||||||||||||
| Chiang, 2012 [45] | Porcine | x | x | x | x | x | x | ||||||||||||||||||
|
| |||||||||||||||||||||||||
| Frauchiger, 2018 [52] | Bovine | x | x | x | x | x | x | x | |||||||||||||||||
|
| |||||||||||||||||||||||||
| Guterl, 2014 [21] | Bovine | x | x | x | x | x | x | x | x | x | |||||||||||||||
|
| |||||||||||||||||||||||||
| Hegewald, 2015 [47] | Sheep | x | x | x | x | x | x | x | |||||||||||||||||
|
| |||||||||||||||||||||||||
| Heuer, 2008 [59] | Bovine | x | x | x | x | x | x | x | x | x | x | ||||||||||||||
|
| |||||||||||||||||||||||||
| Kranenburg, 2012 [62] | Canine | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||
|
| |||||||||||||||||||||||||
| Ledet, 2009 [49] | Sheep | x | x | x | x | x | x | x | |||||||||||||||||
|
| |||||||||||||||||||||||||
| Likhitpanichkul, 2015 [53] | Bovine | x | x | x | x | x | x | x | x | x | x | ||||||||||||||
|
| |||||||||||||||||||||||||
| Long, 2016 [55] | Bovine | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
|
| |||||||||||||||||||||||||
| Long, 2018 [56] | Bovine | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
|
| |||||||||||||||||||||||||
| Reitmar, 2012 [64] | Sheep | x | x | x | x | x | x | x | x | x | |||||||||||||||
|
| |||||||||||||||||||||||||
| Rickers, 2018 [68] | Porcine | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||
|
| |||||||||||||||||||||||||
| Sloan, 2017 [51] | Rat tail | x | x | x | x | x | x | x | x | ||||||||||||||||
|
| |||||||||||||||||||||||||
| Vergroessen, 2015 [60] | Goat | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||
|
| |||||||||||||||||||||||||
| Wilke, 2013 [67] | Human | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||
|
| |||||||||||||||||||||||||
| Yang, 2016 [50] | Porcine | x | x | x | x | x | x | x | |||||||||||||||||
Table 2:
Key findings of the included studies on annular repair testing.
| Citation | Key Finding |
|---|---|
| Ahlgren, 2000 [22] | Direct repair of an annular incision does not significantly alter healing strength of an intervertebral disc |
| Bateman, 2016 [40] | Promising results associated with annular defect closure with a porcine model using 2-0 non-absorbable suture |
| Borde, 2015 [41] | High density collagen gels may be effective at restoring mechanical function of injured discs |
| Bostelman, 2017 [66] | Annular closure device restored pressure response of human cadaver discs after discectomy |
| Bron, 2010 [42] | There were mixed results with an annular closure device in a goat intervertebral disc model |
| Chiang, 2011 [44] | A modified purse string suture technique can seal a damaged anular defect in a porcine lumbar spine |
| Chiang, 2012 [45] | A modified purse-string suture technique generates higher contact pressure than other suture techniques |
| Frauchiger, 2018 [52] | Geinipin-enhanced fibrin hydrogel and an engineered silk scaffold may be used for a intervertebral disc repair |
| Guterl, 2014 [21] | FibGen gel offers promise for a sealant to repair annulus fibrosus defects |
| Hegewald, 2015 [47] | Bio-integrative annular implant shows promise as a mechanical barrier in an ovine model |
| Heuer, 2008 [59] | A promising method for annular repair may be cyanoacrylate glue with suture |
| Kranenburg, 2012 [62] | A nucleus pulposus prosthesis and suture annular repair helped restore the biomechanical properties of an injured intervertebral disc |
| Ledet, 2009 [49] Likhitpanichkul, 2015 |
Using small intestinal submucosa for “patch and plug” repair of annular defects helped maintain hydration and assist in functional recovery of an injured disc |
| [53] | Injectable Fib-Gen may be able to seal annular fibrosus defects |
| Long, 2016 [55] | Fibrin-genipin is an easily deliverable adhesive that can fill an irregularly-shaped annular defects |
| Long, 2018 [56] | Trimethylene carbonate adhesives performed well during in-vitro and in-situ testing as an annular repair material |
| Reitmar, 2012 [64] | Hydrogels that mimic mechanical behavior of a native nucleus may fail to restore the mechanical behavior of an intervertebral disc |
| Rickers, 2018 [68] | Repairing an annular defect with polycaprolactone scaffold improves biomechanical behavior |
| Sloan, 2017 [51] | An injectable riboflavin cross-linked high density collagen gel combined with NP repair with hyaluronic acid hydrogel increased nucleus pulposus hydration |
| Vergroessen, 2015 [60] | Isocyanate-terminated glue increases the force at which nucleus pulposus protrusion occurs |
| Wilke, 2013 [67] | Created a human herniation model and found promising results with the Barricaid annular closure device |
| Yang, 2016 [50] | A modified purse-string suture with an annular graft may increase the integrity of an annulus fibrosis after a punch injury |
Region of spine:
Cervical spine testing was done in 2 studies, lumbar spines were tested in 12 studies and 8 studies used tails within their experiment. The most common animal model used was bovine.
Type of annular defect:
Four experiments used two different methods to create annular defects. The smallest defects noted were in rat tails where defects were less than 1mm in length. Canine, porcine and goat models had approximately the same sized defects of 2–4mm in length. Bovine tails and sheep models required slight larger annular defects between 2–5mm in length. The largest defects were used in the two models using human lumbar spines[66, 67]. Nine experiments used a box defect to model an annular injury. The defects created in the annulus within each study are outlined in Table 3.
Table 3:
The range of annular defects created for biomechanical models.
| Study | Model | Size of defect | Shape of annular defect | Tissue removed |
|---|---|---|---|---|
| Ahlgren, 2000 [22] | Sheep | 5mm | Transverse incision | Partial discectomy |
| Sheep | 5mm × 5mm | Cruciate incision | Partial discectomy | |
| Sheep | 5mm × 3mm | Rectangular box | Partial discectomy | |
| Hegewald, 2015 [47] | Sheep | 3.5mm × 3.5mm | Square box | |
| Ledet, 2009 [49] | Sheep | 4mm × 8mm | Rectangular box | |
| Reitmar, 2012 [64] | Sheep | 4mm | Incision | No discectomy or partial discectomy |
| Bateman, 2016 [40] | Porcine | Vertical incision | ||
| Chiang, 2012 [45] | Porcine | 4mm | Stab incision | |
| Chiang, 2011 [44] | Porcine | 4mm | Transverse incision | |
| Yang, 2016 [50] | Porcine | 3mm × 4mm × 11mm |
Rectangular box | |
| Rickers, 2018 [68] | Porcine | 3mm | Biopsy punch | |
| Borde, 2015 [41] | Rat tail | 0.5 mm | 21 gaude needle | |
| Rat tail | 1mm × 1mm | Square box | ||
| Sloan, 2017 [51] | Rat tail | 1mm × 1mm | Square box | Discectomy |
| Frauchiger, 2018 [52] | Bovine | 2mm | Punch biopsy | |
| Guterl, 2014 [21] | Bovine | 3mm | Punch biopsy | |
| Likhitpanichkul, 2015 [53] | Bovine | 4.5mm × 4.5mm | Square box | |
| Long, 2016 [55] | Bovine | 8mm depth | Cruciate incision | |
| Bovine | 5mm | Punch biopsy | 30% of nucleus removed | |
| Long, 2018 [56] | Bovine | 5mm | Punch biopsy | 25% nucleus removed |
| Bovine | 4mm | Punch biopsy | 150–170 mg nucleus/annulus removed | |
| Heuer, 2008 [59] | Bovine | Oblique incision | Nucleotomy | |
| Bron, 2010 [42] | Goat | 3mm | Circular defect | |
| Vergroessen, 2015 [60] | Goat | 2.4mm | Punch incision | |
| Kranenburg, 2012 [62] | Canine | 3–4mm | Stab incision | Nucleotomy |
| Bostelman, 2017 [66] | Human | 6mm × 10mm | Box incision | |
| Wilke, 2013 [67] | Human | 6mm × 10mm | Box incision |
Apparatus:
Standardized equipment refers to a testing protocol that uses commercially available mechanical testing machines. For example, Sloan et al. used an Enduratec Elf 3200 mechanical testing frame (BOSE, Eden Prarie, MN), while Rickers et al. used an Instron machine (Norwood, MA) [51, 68]. In contrast, Frauchiger et al. used a custom-designed apparatus to place loads over the intervertebral disc[52]. In all, 9/22 studies utilized a custom-built apparatus to perform biomechanical testing. Of note, Vergrosen et al. used a standard testing apparatus to identify the ultimate strength of their device and also used a custom apparatus in order to identify the endurance behavior of their device[60].
Mode of control:
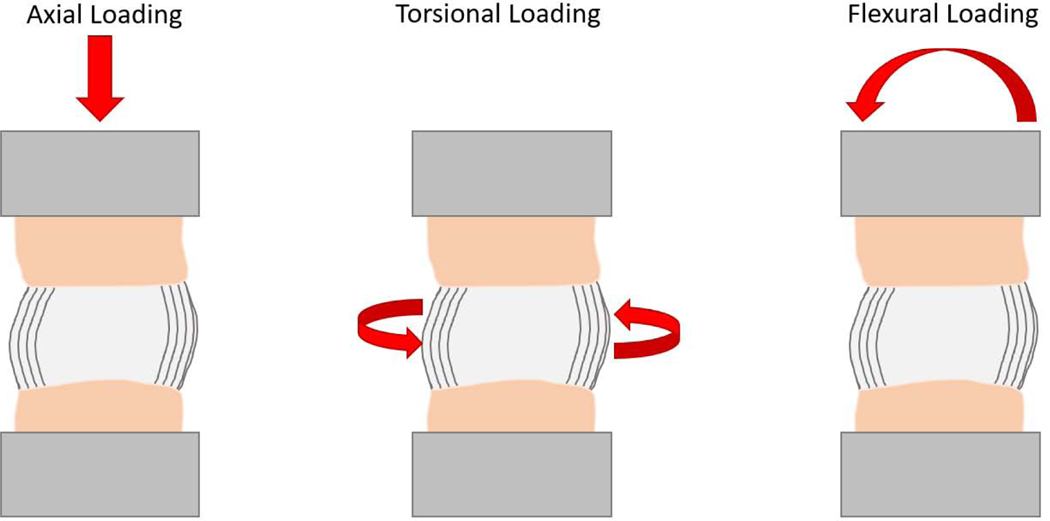
Displacement-controlled systems are programmed to move the loading fixture a certain distance irrespective of the force being applied. Load-controlled systems apply a prescribed load irrespective of the distance the loading fixture travels. Displacement and loadcontrol can be implemented in different directions relative to the anatomy of the cadaveric tissue (Figure 2, Table 1). Axial loading was the most common control mode used in the literature reviewed with load control being used in 15/22 studies, displacement control used in 4/22 studies and 2/22 studies performing both axial load and displacement control testing. Torsional loading around the body of the disc were mainly implemented by applying a defined torsion angle and measuring the required torque to reach that angle (6/22 studies). In the studies that performed flexion testing, the spinal segments were flexed to a defined flexion angle in 7/22 studies while load controlled flexion of spinal segments to a specified torque was used in 3/22 studies.
Figure 2:
Illustration of different modes of control on a segment of the spine. The beige area represents the vertebral body, black lines the annulus and the light grey the nucleus pulposus. The dark grey is the fixture through which force is being applied to the specimen.
Dynamic vs. Static:
Dynamic vs static testing refers to a single load/ displacement was applied (static) or a number of sequential cycles of load/ displacement were applied (cyclic). The majority of studies used a static force (21/22 studies). A significant portion of studies used a dynamic force on the repaired segment (8/22 studies). Seven of the experiments utilized both dynamic and static testing protocols.
Primary outcome metric:
The most common outcome was the force/pressure required for herniation of the disc (15/22 studies), Table 4. Five tests involved needle pressurization of the disc space until material extrusion. Fourteen studies included data on a failure load which represents the magnitude of the force required to disrupt the disc or cause device failure. One study described the failure load for suture loops[43]. One test involved indenting a material with a punch until failure[21]. The values reported and how the disc herniation was detected is outlined in Table 4. The pressure to cause disc herniation ranged from 330 kPa to 10,253 kPa across species. Of note, there was no standardized unit to define the failure force. Certain studies reported failure pressure while others reported failure force. Most often, disc herniation was observed by the human eye, and as such was subjective. In a portion of studies, failure load was also defined as endplate fracture. In 8 studies, a load was placed over the disc space to cause herniation[42, 55, 56, 59, 60, 67, 68]. Three of these studies involved an axial load while 5 studies placed an eccentric load over the disc space with an angled moment. Another common test performed was range of motion before and after disc repair, reported in 8 studies.
Table 4:
Summary of articles that contain failure mechanical testing data for disc repair material. Tests have been grouped together in order to compare how different studies measured and produced a failure load on a repaired disc.
| Author | Model | Mechanism of Failure | How did they measure failure? | Force for failure |
|---|---|---|---|---|
| Ahlgren, 2000 [22] | Sheep | Injected disc and measured pressure at failure | Leakage at annular defect by observer | 330 kPa to 1410 kPa |
| Hegewald, 2015 [47] | Sheep | Injected disc and measured pressure at failure | Leakage at annular defect until contrast leakage by fluoroscopy | 530 kPa |
| Ledet, 2009 [49] | Sheep | Injected disc and measured pressure at failure | Leakage at annular defect until contrast leakage by observer | 5,050 kPa |
| Chiang, 2012 [45] | Porcine | Injected disc and measured pressure at failure | Leaked fluid from annular defect by observer | 920–1,700 kPa |
| Chiang, 2011 [44] | Porcine | Failure of disc/repair with repeated axial compression in static compression and cyclic compressive cycles | Leaked content of disc detected by observer | 2,917 N |
| Yang, 2016 [50] | Porcine | Injected disc and measured pressure at failure | Leakage at annular defect until contrast leakage by observer | 1,020 – 4,870 kPa |
| Long, 2016 [55] | Bovine | 20 cycles of from low flexion to 19 deg flexion until herniation detected by a camera after 25% of NP removed | Detected via camera through the loading process | Maximum torque 10.3 +/− 5.9 Nm |
| Long, 2018 [56] | Bovine | Flexion (0.50 MPa) extension (0.25 MPa) of disc space until herniation detected by a camera | Detected via camera through the loading process | 4,500–5,900 kPa |
| Heuer, 2008 [59] | Bovine | Cyclic off-center loading of 100–600N | Observed herniation of disc material | 3400–16,900 cycles until herniation |
| Bron, 2010 [42] | Goat | Axial compression until extrusion of disc material | Observed herniation of disc material | ~1000N to 4000N |
| Vergroessen, 2015 [60] | Goats | 5 deg left lateral flexion until herniation | Observed herniation of disc material | 5,500–7,400 kPa |
| Wilke, 2013 [67] | Human Lumbar | Sinusoidal load until disc failure or 100,000 cycles | Observed herniation of disc material | 100–600 N |
| Rickers, 2018 [68] | Porcine | Axial compression until failure or 4000N | Observed leakage of disc material | 300–3100 N |
| Guterl, 2014 [21] | Bovine | Press fit repair material in annular defect and indented at 0.01mm/s | When repair material fell out of press fit defect | 81–146% failure strain |
Hydrogel Experiments:
Eight studies examined the performance of a hydrogel for disc repair, see Table 5. Five of these studies employed the hydrogel as an annular repair[21, 41, 52, 53, 55]. Three studies examined biomechanical performance of a nucleus replacement device with a concurrent annular repair strategy included within their experiment[51, 62, 64]. Four of the studies examined the performance of a fibrin-genipin material for annular repair[21, 52, 53, 55]. Two studies used a hyaluronic acid derived hydrogel [51, 64]. Only one of the hydrogels used for disc repair was based off a synthetic molecule[62]. The rest of the hydrogels were derived from organic molecules. Three of the experiments fixated the hydrogel within the disc space with suture to prevent re-herniation. Five of the studies relied on the hydrogel to be “self-adhesive” and to be solidly placed into the disc defect without fixation augmentation devices.
Table 5:
Results from studies that included a hydrogel as a method of fixing an annular defect in an intervertebral disc.
| Author, Year | Model | Tested mechanical properties | Hydrogel Description | Attachment of Hydrogel | Origin - synthetic or organic | Crosslinking |
|---|---|---|---|---|---|---|
| Frauchiger, 2018 [52] | Bovine | Stress strain relationship | Genipin-enhanced fibrin hydrogel | Injected then covered with silk-fleece composite | Organic - human fibrinogen | Genipin crosslinks |
| Likhitpanichkul, 2015 [53] | Bovine | Disc height, Compressive stiffness, Tensile stiffness, Torsional stiffness, Torque range, Stress relaxation time | Fibrin-genipin hydrolgel | Self-adhesive to intervertebral disc | Organic - human fibrinogen | Genipin crosslinks |
| Long, 2016 [55] | Bovine | Torque stiffness, Torque range, On and off axis stiffness, Maximum torque, Maximum nominal axial stress, Maximum rotation angle, Range of motion, Herniation detected by camera | Fibrin-genipin hydrolgel with poly(trimethyl carbonate) and a polyurethane membrane | Self adhesive and self-adhesive/sutured into place | Organic - human fibrinogen | Genipin crosslinks |
| Guterl, 2014 [21] | Bovine | Adhesion strength, Adhesive failure | Genipin, Genipin with fibronectin, genipin with collagen and uncrosslinked fibrin gel | Self-adhesive to intervertebral disc | Organic - human fibrinogen | Genipin crosslinks |
| Kranenburg, 2012 [62] | Canine | Range of motion, Range of angular dislpacement, Disc height, Stiffness (elastic modulus) | 4-IEMA, NVP, HEMA and AIBN mixed together then heated for polymerization | Layered closure of muscle/fascia with an annular suture repair (size 4-0) | Synthetic (4-IEMA, NVP, HEMA, AIBN) | Cyclic heating |
| Reitmair, 2012 [64] | Sheep | Intradiscal pressure, Disc height Change, Compression stiffness | Two hydrogels, DDAHA which is an’ amidic hyaluronic acid derivative and iGG-MA which is an extracellular microbial polysacharide | Annular repair with adhesive glue and sutures | Organic - hyarluronic acid and microbial polysacharide | Gellan gum used for crosslinking for iGG-MA |
| Sloan, 2017 [51] | Rat Tail | Hydraulic permeability, instantaneous modulus, equilibrium modulus | HYADD4®, hydrogel based on hyaluronic acid derivative | Collagen patch used to fill annular defect through which NP replacement is implanted | Organic - hyaluronic acid derivative | No crosslinking |
| Borde, 2015 [41] | Rat Tail | Effective equilibirum modulus, Effective instantaneous modulus, Effective hydraulic permeability | High density collagen gel with riboflavin | Injected into annular defect - self-attachment | Organic - Collagen gel | Ribofloxacin crosslinked |
The mechanical properties (e.g. stiffness, hydraulic permeability) and function (e.g. range of motion, disc height change) of these hydrogels were tested in 6/8 studies. A measure of the failure of the repair material was only performed in 2/8 studies. In the two studies that performed failure testing, Guterl et al. performed a pushout test of the hydrogel from an annular defect[21]. In situ failure testing was only performed by Long et al. looking at failure of the hydrogel repair in torsion and flexion[55].
Mechanical repairs/barriers for annular defect:
Several studies examined the performance of mechanical barriers for annular repair. Sutures were used either alone or in contrast to other repair strategies (i.e. adhesive glue or hydrogel) in 8 separate studies. Three studies utilized pressure-volume testing where a quantity of fluid was injected within the intervertebral disc to determine the pressure required for disc herniation. Failure load ranged from 1500 kPa – 5650 kPa[22, 45, 50]. Higher failure loads were tolerated in the porcine model as compared to the sheep model.
A custom designed mechanical barrier that included an endplate fixation device was tested in two separate studies[66, 67]. Failure loads and range of motion testing were performed for each experiment where a box annular defect was created and treated with the annular closure device. Schematics from both studies show custom designs for testing each mechanical property.
Fibrin sealant and adhesive glues were used to close an annular defect across three separate animal models with varying mechanical properties tested in each[59, 60, 64]. Long et al. repaired an annular defect with copolymers of polyethylene glycol with varying end-groups and tested varying mechanical properties in a bovine tail[56]. Hegewald et al. tested sheep lumbar spine for the performance of a bio-integrative annulus implant consisting of polyglycolic acid/polyvinylidene fluoride. The failure load for pressure volume testing for the device was 530 kPa[47].
Experimental controls:
Experimental controls were utilized in different manners across studies. Only 3 studies did not use a control. Controls were either defined as an injured disc without repair or a non-injured disc subjected to the same biomechanical testing protocol. All studies which used a control included an injured disc without repair of any kind (19 studies) and the majority of experiments used an uninjured specimen as a control (16/22 studies). In two studies, the control discs and the disc spaces injured/repaired were within the same lumbar spine during testing[22, 49]. Both studies used pressure-volume testing for testing the integrity of the disc space.
Iterations in testing:
Several studies used custom designed models that were modified over many years in their goal of mechanically testing annular closure devices. Ahlgren et al. and Ledet et al. modeled their biomechanical process on the same study by Panjabi et al.[22, 49, 69]. These studies used the same model of injection of fluid within a disc space using different annular repair techniques. Within the original Panjabi et al. study 84 fresh cadaveric specimens were injected with a contrast agent and the maximum/intrinsic pressure was monitored. Yang et al. injected material into a disc to test the integrity of their disc repair device but referenced a separate study by Schechtman et al. when describing their technique[50, 70]. The Schetman et al. and Yang et al. studies monitored disc pressure from above the disc space through the intervertebral body[50, 70]. In a series of studies, Borde et al., Sloan et al. and Bowles et al. described similar mechanical testing protocols for rat tails[41, 51, 71]. They compared equilibrium modulus, effective instantaneous modulus and hydraulic permeability of their collagen gel and/or hydrogel. During tests, a 5% compressive strain was applied in a stepwise fashion until 20% of disc height was reached. The studies by Sloan et al. and Borde et al. are based on an initial study of a nucleus replacement device from the same group[71]. The testing protocol did not seem to change dramatically between studies from 2011–2017.
The biomechanical studies from one group demonstrated significant iterations in terms of biomechanical testing over the span of several years. Guterl et al. used an annular punch method to test adhesion strength of the fibrin-genipin repair material[21]. The next included study from this group included a more complex set of biomechanical testing[53]. The authors of Likhitpanichkul et al. utilized an axial and torsional method of applying force to a motion segment and measured disc height changes, stiffness, range of motion and hysteresis of the repaired disc space[53]. A similar range of mechanical parameters and cycles of force were used to stress the disc repair device in the next study from the group[55]. The most recent study that was included from the group used the same protocol of a preload with cyclic compressive cycles over a motion segment[56]. This last study by Long et al. added an additional data point of a failure mode test with cyclic compression of the disc space until extrusion of a disc as seen by video[56].
Discussion
Our systematic review delineates the current body of literature surrounding the biomechanical performance of annular repair material. We found 22 articles that matched our inclusion criteria. Despite our thorough review, we were unable to delineate common test parameters to simplify comparisons of studies, because there was too much variation in testing methodology and reported results. Given the multidirectional, dynamic loads to which the intervertebral disc is subjected[72], and clinical concern over the severe consequences of failure, we advocate for the development of a clinically relevant, standardized, pre-clinical test-to-failure system, which can be used for the mechanical screening of candidate materials for AF repair.
Our review builds upon previously published review articles on the biomechanics of the spine. Stokes et al. synthesized data on the pathophysiology of disc degeneration with a focus on how abnormal loading, including immobilization, can cause adaptive changes within an intervertebral disc[73]. Weber et al. described the molecular changes that occur with disc degeneration and regeneration as well as offering a broad overview of hydrogels and annular repair devices[74]. Inoue et al. reviewed the biomechanical changes associated with disc degeneration[75], while both Alini et al. and Long et al. reviewed biomechanical requirements of annular repair devices[28, 76]. We have built upon these reviews by specifically analyzing biomechanical test systems and approaches as applied to annular repair devices to provide a unique perspective on the challenges that clinicians face when trying to decipher the various test systems and results presented in literature. For instance, unlike other systematic reviews, it is difficult to create a useful forest plot of results from each study given the wide variety of primary outcome measures, species tested, testing apparatus, etc. used among all the studies included within the review. The authors acknowledge, however, that a limitation of our study is that we are not outlining/defining deficiencies of each of our included studies.
Although modeling a complex clinical situation like a disc herniation is difficult, it is a particularly important consideration when attempting to create a defect across which the biomechanical performance of an annular repair device can be reliably characterized. The degeneration of the disc itself is in large part cause by genetic factors which make some disc herniations destine to herniate[77]. Disc degeneration may also be induced from prolonged exposure to whole body vibration[78]. To circumvent the insurmountable challenges of replicating a lifetime of vibration on a disc space or the intrinsic genetic factors, we found that defects were created in a wider variety of ways using punch biopsies, needle punctures, transverse incisions, and box incisions. With such varied incisions, the mechanical effect on candidate repair devices is likely to reflect that variability. Establishing a simple and reproducible defect to both create an annular defect and extrude an intervertebral disc will be vital to allow for cross comparison of studies.
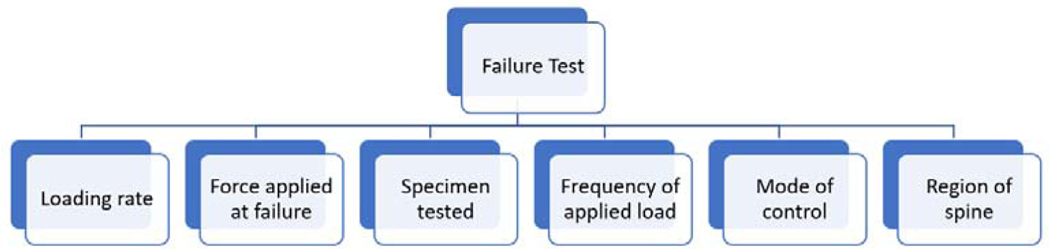
The proximity of nerve roots and thecal sac posteriorly as well as the great vessels anteriorly makes it critically important to ensure a disc repair device does not fail under the most severe biomechanical environment. Any displacement or fragmentation of the device could mean dire consequences for a patient. For instance, cases of posterior migration of an interbody cage after a transforaminal lumbar interbody fusion cage insertion which resulted in a return to the operating room and nerve injury, have been reported[79]. Given the potentially severe consequences of mechanical failure, and the risks of translating devices to clinical use without preempting all possible modes of failure[80, 81], pre-clinical biomechanical testing that mimics expected modes of failure is crucial. Therefore, it is not surprising that the most commonly tested parameter was failure force for a device. Unfortunately, cross comparisons of the results from testing-to-failure is not straightforward. A myriad of factors and relationships that must be delineated in order to understand what a failure force means in the context of an experiment, Figure 3. Each of the factors listed must be controlled for within an experiment to understand how one group’s results compare to another group’s results with similar or different annular repair device.
Figure 3:
The factors that should be clearly defined in a failure test. If a failure test is created, scaling factors must be outlined if a different species or if a different region of the spine is tested.
A cross section of standard, commercailly available and customized test systems were used to test the biomechanical properties of annular repair devices. Thirteen studies used a standard testing apparatus and 9 studies used a custom built apparatus. It is valid to use a custom apparatus for these biomechanical tests as long as the description provided within a study is clear enough to be replicated. Both Long et al. and Bostelman et al. have detailed “Methods” sections that outline pieces of equipment required and how to setup thes testing protocol[56, 66]. Photos of setups also help guide future researchers to replicate testing conditions[66]. In our detailed reading of the methodology of all 9 of these apparatuses, 2/9 of these tests we felt lacked enough clarity to reliably create a replicate apparatus[47, 60]. Other studies outlined testing apparatuses that were complex and would likely require significant time/resources from an experienced mechanical engineer[52, 59]. Whatever testing becomes uniformly accepted as a “gold standard” must be reproducible, validated and replicated in multiple labs and allow researchers to easily setup and administer.
Amongst all studies substantial variability in species tested and the region of the spine tested was noted. As described by Alini et al. scaling of results from animal species and different regions of the spine is not straightforward for a number of reasons[76]. These include the significant variation in the geometry of discs, patterns of annular fibers, orientation of stabilizing facet joints and the biology of discs. Future research is required to understand how one biomechanical test performs on different models and different regions of the spine. Moreover, as advancements in cell therapies for intervertebral disc degeneration continue further research will also be required in the biomechanical behavior of these repaired discs. Reconstituting native tissue may be the eventual goal but testing whether this newly created tissue is comparable to native disc spaces will need to validated[82]. We strongly encourage scientists experimenting with these cell-based therapies pay special care to understand and test out the biomechanical properties of their therapy to ensure safe usage within humans.
In summary, while there have been extensive scientific advancements in design of compounds/barriers to repair a disc, the lack of standardized, reproducible biomechanical test systems that are widely accepted as a benchmark for disc repair material is a barrier to clinical translation. The authors propose that a simplified and scientific based platform for standardized testing of disc repair devices be designed for both animal and human cadaver spines. Widespread use of this standardized protocol would allow movement from bench top research to eventual clinical trials for promising annular repair products.
Clinical Significance:
This literature review provides a summary of pre-clinical testing of annular repair devices for clinicians to properly evaluate the safety/efficacy of developing technology designed to repair annular defects after disc herniations.
Acknowledgments
There was no financial support used for the authoring of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K. Long-Term Outcomes of Standard Discectomy for Lumbar Disc Herniation: A Follow-Up Study of More Than 10 Years. 2001;26(6):652–7. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ballestros K, Echko M, et al. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. Jama. 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. [DOI] [PubMed] [Google Scholar]
- 5.Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. The Journal of bone and joint surgery British volume. 2005;87-B(1):62–7. [PubMed] [Google Scholar]
- 6.Battié MC, Videman T, Parent E. Lumbar Disc Degeneration: Epidemiology and Genetic Influences. 2004;29(23):2679–90. [DOI] [PubMed] [Google Scholar]
- 7.Hansson E, Hansson T. The cost–utility of lumbar disc herniation surgery. European Spine Journal. 2007;16(3):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. The Spine Journal. 2008;8(1):8–20. [DOI] [PubMed] [Google Scholar]
- 9.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29(1):79–86. [DOI] [PubMed] [Google Scholar]
- 10.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. Jama. 2003;290(18):2443–54. [DOI] [PubMed] [Google Scholar]
- 11.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. Jama. 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 12.Carragee EJ, Han MY, Suen PW, Kim D. Clinical Outcomes After Lumbar Discectomy for Sciatica: The Effects of Fragment Type and Anular Competence. 2003;85(1):102–8. [PubMed] [Google Scholar]
- 13.Virk SS, Diwan A, Phillips FM, Sandhu H, Khan SN. What is the Rate of Revision Discectomies After Primary Discectomy on a National Scale? Clinical orthopaedics and related research. 2017;475(11):2752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BUTTERMANN GR. TREATMENT OF LUMBAR DISC HERNIATION: EPIDURAL STEROID INJECTION COMPARED WITH DISCECTOMYA PROSPECTIVE, RANDOMIZED STUDY. 2004;86(4):670–9. [PubMed] [Google Scholar]
- 15.Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and Nonsurgical Management of Sciatica Secondary to a Lumbar Disc Herniation: Five-Year Outcomes From the Maine Lumbar Spine Study. 2001;26(10):1179–87. [DOI] [PubMed] [Google Scholar]
- 16.Drazin D, Ugiliweneza B, Al-Khouja L, et al. Treatment of Recurrent Disc Herniation: A Systematic Review. Cureus. 2016;8(5):e622-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heliövaara M, Impivaara O, Sievers K, et al. Lumbar disc syndrome in Finland. 1987;41(3):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaston P, Marshall RW. Survival analysis is a better estimate of recurrent disc herniation. The Journal of bone and joint surgery British volume. 2003;85(4):535–7. [DOI] [PubMed] [Google Scholar]
- 19.Fields AJ, Rodriguez D, Gary KN, Liebenberg EC, Lotz JC. Influence of biochemical composition on endplate cartilage tensile properties in the human lumbar spine. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32(2):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS Prize Winner: The Anatomy of Failure in Lumbar Disc HerniationAn In Vivo, Multimodal, Prospective Study of 181 Subjects. 2013;38(17):1491–500. [DOI] [PubMed] [Google Scholar]
- 21.Guterl CC, Torre OM, Purmessur D, et al. Characterization of mechanics and cytocompatibility of fibrin-genipin annulus fibrosus sealant with the addition of cell adhesion molecules. Tissue engineering Part A. 2014;20(17–18):2536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000;25(17):2165–70. [DOI] [PubMed] [Google Scholar]
- 23.Choy WJ, Phan K, Diwan AD, Ong CS, Mobbs RJ. Annular closure device for disc herniation: metaanalysis of clinical outcome and complications. BMC musculoskeletal disorders. 2018;19(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markolf KL, Morris JM. The structural components of the intervertebral disc. A study of their contributions to the ability of the disc to withstand compressive forces. The Journal of bone and joint surgery American volume. 1974;56(4):675–87. [PubMed] [Google Scholar]
- 25.Chan SCW, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. European Spine Journal. 2011;20(11):1796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neidlinger-Wilke C, Galbusera F, Pratsinis H, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. European Spine Journal. 2014;23(3):333–43. [DOI] [PubMed] [Google Scholar]
- 27.Gregory DE, Callaghan JP. An examination of the mechanical properties of the annulus fibrosus: The effect of vibration on the intra-lamellar matrix strength. Medical Engineering & Physics. 2012;34(4):472–7. [DOI] [PubMed] [Google Scholar]
- 28.Long RG, Torre OM, Hom WW, Assael DJ, Iatridis JC. Design Requirements for Annulus Fibrosus Repair: Review of Forces, Displacements, and Material Properties of the Intervertebral Disk and a Summary of Candidate Hydrogels for Repair. Journal of biomechanical engineering. 2016;138(2):021007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanayama M, Tadano S, Kaneda K, Ukai T, Abumi K, Ito M. A cineradiographic study on the lumbar disc deformation during flexion and extension of the trunk. Clinical biomechanics (Bristol, Avon). 1995;10(4):193–9. [DOI] [PubMed] [Google Scholar]
- 30.Nagel TM, Zitnay JL, Barocas VH, Nuckley DJ. Quantification of continuous in vivo flexion–extension kinematics and intervertebral strains. European Spine Journal. 2014;23(4):754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iatridis JC, MacLean JJ, Roughley PJ, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. The Journal of bone and joint surgery American volume. 2006;88 Suppl 2:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33(6):588–96. [DOI] [PubMed] [Google Scholar]
- 33.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30(1):5–14. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmocker A, Khoushabi A, Frauchiger DA, et al. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. 2016;88:110–9. [DOI] [PubMed] [Google Scholar]
- 37.Coric D, Mummaneni PV. Nucleus replacement technologies. 2008;8(2):115. [DOI] [PubMed] [Google Scholar]
- 38.Lee T, Lim TH, Lee SH, Kim JH, Hong JJJoOR. Biomechanical function of a balloon nucleus pulposus replacement system: A human cadaveric spine study. 2018;36(1):167–73. [DOI] [PubMed] [Google Scholar]
- 39.Balkovec C, Vernengo J, McGill SM. The use of a novel injectable hydrogel nucleus pulposus replacement in restoring the mechanical properties of cyclically fatigued porcine intervertebral discs. Journal of biomechanical engineering. 2013;135(6):61004–5. [DOI] [PubMed] [Google Scholar]
- 40.Bateman AH, Balkovec C, Akens MK, et al. Closure of the annulus fibrosus of the intervertebral disc using a novel suture application device-in vivo porcine and ex vivo biomechanical evaluation. The spine journal : official journal of the North American Spine Society. 2016;16(7):889–95. [DOI] [PubMed] [Google Scholar]
- 41.Borde B, Grunert P, Hartl R, Bonassar LJ. Injectable, high-density collagen gels for annulus fibrosus repair: An in vitro rat tail model. Journal of biomedical materials research Part A. 2015;103(8):2571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bron JL, van der Veen AJ, Helder MN, et al. Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. European Spine Journal. 2010;19(8):1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buser Z, Kuelling F, Liu J, et al. Biological and biomechanical effects of fibrin injection into porcine intervertebral discs. Spine. 2011;36(18):E1201–9. [DOI] [PubMed] [Google Scholar]
- 44.Chiang CJ, Cheng CK, Sun JS, Liao CJ, Wang YH, Tsuang YH. The effect of a new anular repair after discectomy in intervertebral disc degeneration: an experimental study using a porcine spine model. Spine. 2011;36(10):761–9. [DOI] [PubMed] [Google Scholar]
- 45.Chiang YF, Chiang CJ, Yang CH, et al. Retaining intradiscal pressure after annulotomy by different annular suture techniques, and their biomechanical evaluations. Clinical biomechanics (Bristol, Avon). 2012;27(3):241–8. [DOI] [PubMed] [Google Scholar]
- 46.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2007;16(11):1892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegewald AA, Medved F, Feng D, et al. Enhancing tissue repair in annulus fibrosus defects of the intervertebral disc: analysis of a bio-integrative annulus implant in an in-vivo ovine model. Journal of tissue engineering and regenerative medicine. 2015;9(4):405–14. [DOI] [PubMed] [Google Scholar]
- 48.Malhotra NR, Han WM, Beckstein J, Cloyd J, Chen W, Elliott DM. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine. 2012;37(18):E1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ledet EH, Jeshuran W, Glennon JC, et al. Small intestinal submucosa for anular defect closure: long-term response in an in vivo sheep model. Spine. 2009;34(14):1457–63. [DOI] [PubMed] [Google Scholar]
- 50.Yang CH, Chiang YF, Chen CH, Wu LC, Liao CJ, Chiang CJ. The effect of annular repair on the failure strength of the porcine lumbar disc after needle puncture and punch injury. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2016;25(3):906–12. [DOI] [PubMed] [Google Scholar]
- 51.Sloan SR Jr., Galesso D, Secchieri C, Berlin C, Hartl R, Bonassar LJ. Initial investigation of individual and combined annulus fibrosus and nucleus pulposus repair ex vivo. Acta biomaterialia. 2017;59:192–9. [DOI] [PubMed] [Google Scholar]
- 52.Frauchiger DA, May RD, Bakirci E, et al. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. Journal of functional biomaterials. 2018;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Likhitpanichkul M, Dreischarf M, Illien-Junger S, et al. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. European cells & materials. 2014;28:25–37; discussion −8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Growney Kalaf EA, Pendyala M, Bledsoe JG, Sell SA. Characterization and restoration of degenerated IVD function with an injectable, in situ gelling alginate hydrogel: An in vitro and ex vivo study. Journal of the mechanical behavior of biomedical materials. 2017;72:229–40. [DOI] [PubMed] [Google Scholar]
- 55.Long RG, Burki A, Zysset P, et al. Mechanical restoration and failure analyses of a hydrogel and scaffold composite strategy for annulus fibrosus repair. Acta biomaterialia. 2016;30:116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long RG, Rotman SG, Hom WW, et al. In vitro and biomechanical screening of polyethylene glycol and poly(trimethylene carbonate) block copolymers for annulus fibrosus repair. Journal of tissue engineering and regenerative medicine. 2018;12(2):e727–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmocker A, Khoushabi A, Frauchiger DA, et al. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials. 2016;88:110–9. [DOI] [PubMed] [Google Scholar]
- 58.Thorpe AA, Dougill G, Vickers L, et al. Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function. Acta biomaterialia. 2017;54:212–26. [DOI] [PubMed] [Google Scholar]
- 59.Heuer F, Ulrich S, Claes L, Wilke H-J. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Journal of Neurosurgery: Spine. 2008;9(3):307–13. [DOI] [PubMed] [Google Scholar]
- 60.Vergroesen PP, Bochyn Ska AI, Emanuel KS, et al. A biodegradable glue for annulus closure: evaluation of strength and endurance. Spine. 2015;40(9):622–8. [DOI] [PubMed] [Google Scholar]
- 61.Showalter BL, Elliott DM, Chen W, Malhotra NR. Evaluation of an In Situ Gelable and Injectable Hydrogel Treatment to Preserve Human Disc Mechanical Function Undergoing Physiologic Cyclic Loading Followed by Hydrated Recovery. Journal of biomechanical engineering. 2015;137(8):081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kranenburg HJ, Meij BP, Onis D, et al. Design, synthesis, imaging, and biomechanics of a softness-gradient hydrogel nucleus pulposus prosthesis in a canine lumbar spine model. Journal of biomedical materials research Part B, Applied biomaterials. 2012;100(8):2148–55. [DOI] [PubMed] [Google Scholar]
- 63.Khandaker M, Riahanizad S. Evaluation of Electrospun Nanofiber-Anchored Silicone for the Degenerative Intervertebral Disc. Journal of healthcare engineering. 2017;2017:5283846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reitmaier S, Wolfram U, Ignatius A, et al. Hydrogels for nucleus replacement—Facing the biomechanical challenge. Journal of the mechanical behavior of biomedical materials. 2012;14:67–77. [DOI] [PubMed] [Google Scholar]
- 65.van Heeswijk VM, Thambyah A, Robertson PA, Broom ND. Posterolateral Disc Prolapse in Flexion Initiated by Lateral Inner Annular Failure: An Investigation of the Herniation Pathway. Spine. 2017;42(21):1604–13. [DOI] [PubMed] [Google Scholar]
- 66.Bostelmann R, Steiger HJ, Cornelius JF. Effect of Annular Defects on Intradiscal Pressures in the Lumbar Spine: An in Vitro Biomechanical Study of Diskectomy and Annular Repair. J Neurol Surg A Cent Eur Neurosurg. 2017;78(1):46–52. [DOI] [PubMed] [Google Scholar]
- 67.Wilke HJ, Ressel L, Heuer F, Graf N, Rath S. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine. 2013;38(10):E587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rickers K, Bendtsen M, Le DQS, Veen AJd, Bünger CE. Biomechanical evaluation of annulus fibrosus repair with scaffold and soft anchors in an ex vivo porcine model. SICOT-J. 2018;4:38-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panjabi M, Brown M, Lindahl S, Irstam L, Hermens M. Intrinsic disc pressure as a measure of integrity of the lumbar spine. Spine. 1988;13(8):913–7. [DOI] [PubMed] [Google Scholar]
- 70.Schechtman H, Robertson PA, Broom ND. Failure strength of the bovine caudal disc under internal hydrostatic pressure. Journal of biomechanics. 2006;39(8):1401–9. [DOI] [PubMed] [Google Scholar]
- 71.Bowles RD, Gebhard HH, Härtl R, Bonassar LJ. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proceedings of the National Academy of Sciences. 2011;108(32):13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilke H, Neef P, Hinz B, Seidel H, Claes L. Intradiscal pressure together with anthropometric data--a data set for the validation of models. Clinical biomechanics (Bristol, Avon). 2001;16 Suppl 1:S111–26. [DOI] [PubMed] [Google Scholar]
- 73.Stokes IA, Iatridis JCJS. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. 2004;29(23):2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weber KT, Jacobsen TD, Maidhof R, et al. Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. 2015;8(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inoue N, OrÝas AAEJOC. Biomechanics of intervertebral disk degeneration. 2011;42(4):487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? European Spine Journal. 2008;17(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams MA, Lama P, Zehra U, Dolan P. Why do some intervertebral discs degenerate, when others (in the same spine) do not? 2015;28(2):195–204. [DOI] [PubMed] [Google Scholar]
- 78.Wahlstrom J, Burstrom L, Johnson PW, Nilsson T, Jarvholm B. Exposure to whole-body vibration and hospitalization due to lumbar disc herniation. International archives of occupational and environmental health. 2018;91(6):689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aoki Y, Yamagata M, Nakajima F, Ikeda Y, Takahashi K. Posterior migration of fusion cages in degenerative lumbar disease treated with transforaminal lumbar interbody fusion: a report of three patients. Spine. 2009;34(1):E54–8. [DOI] [PubMed] [Google Scholar]
- 80.Schemitsch EH, Bhandari M, Boden SD, et al. The evidence-based approach in bringing new orthopaedic devices to market. The Journal of bone and joint surgery American volume. 2010;92(4):1030–7. [DOI] [PubMed] [Google Scholar]
- 81.Virk SS, Kocher MS. Adoption of New Technology in Sports Medicine: Case Studies of the GoreTex Prosthetic Ligament and of Thermal Capsulorrhaphy. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2011;27(1):113–21. [DOI] [PubMed] [Google Scholar]
- 82.Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine. 2018;1(4):e1036-e. [DOI] [PMC free article] [PubMed] [Google Scholar]