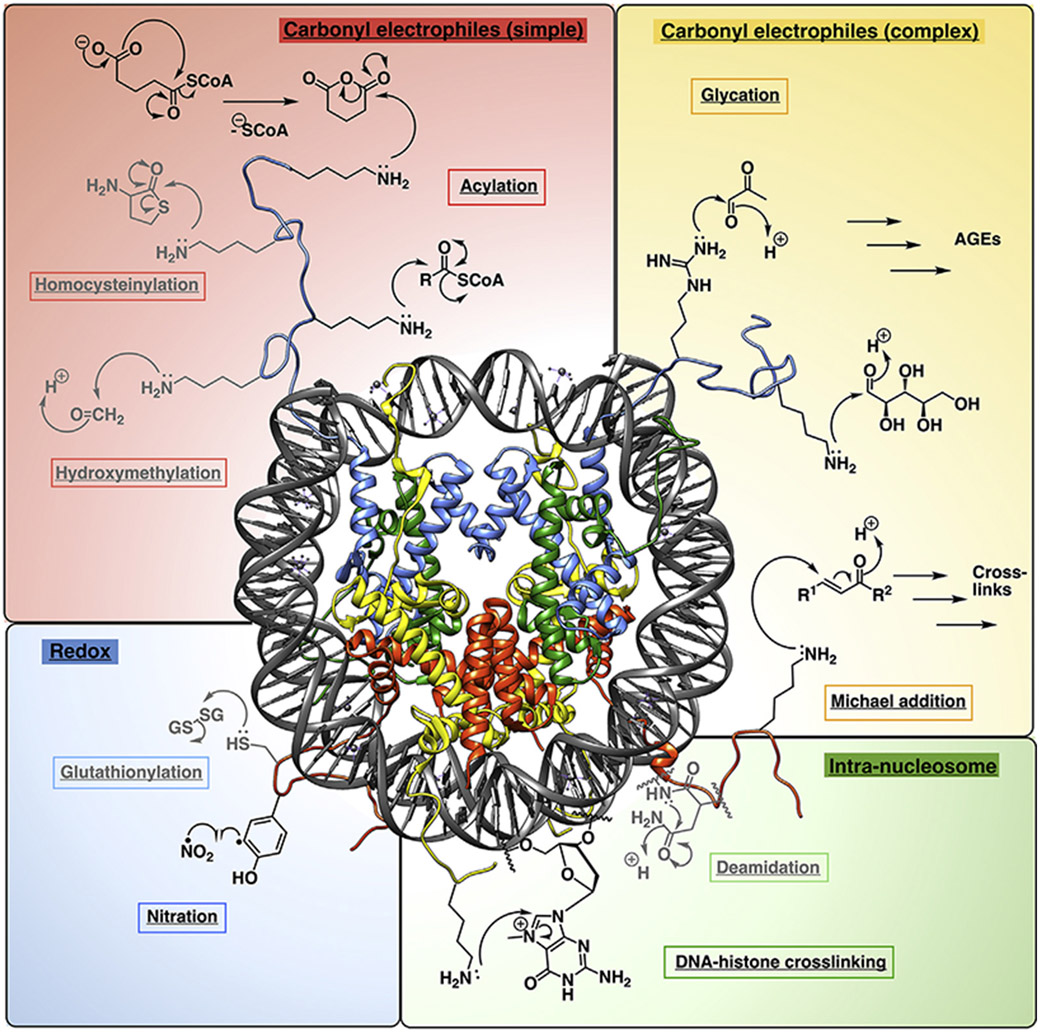
Figure 1. Representative NECM-forming reactions on histones.
Clockwise from top left: reactions with single carbonyl electrophiles including, from top to bottom: acetyl-coenzyme A (coA) derivatives via cyclic intermediates (top) or direct displacement of coA (bottom, R = variable functional group) (acylations), homocysteine (homocysteinylation), and formaldehyde (hydroxymethylation). Top right: reactions with carbonyl electrophiles at multiple carbonyls or distal positions, which give rise to complex mixtures of products. Electrophiles depicted include methylglyoxal (MGO, top), ribose (bottom) (glycation), which both rearrange to form advanced glycation end-products (AGEs) and α,β-unsaturated carbonyls (Michael addition, R1 and R2 = variable functional groups), giving rise to crosslinked products. Bottom right: Intranucleosomal reactions. Electrophiles depicted include asparagine (deamidation) and damaged DNA bases (DNA-histone crosslinking). Bottom left: redox reactions. Species depicted include oxidized glutathione (glutathionylation) and nitrate radical (nitration). Reactions depicted with lysine as the nucleophile may also occur with arginines, and vice-versa. Reactions shown in grey are not discussed in depth in this review.