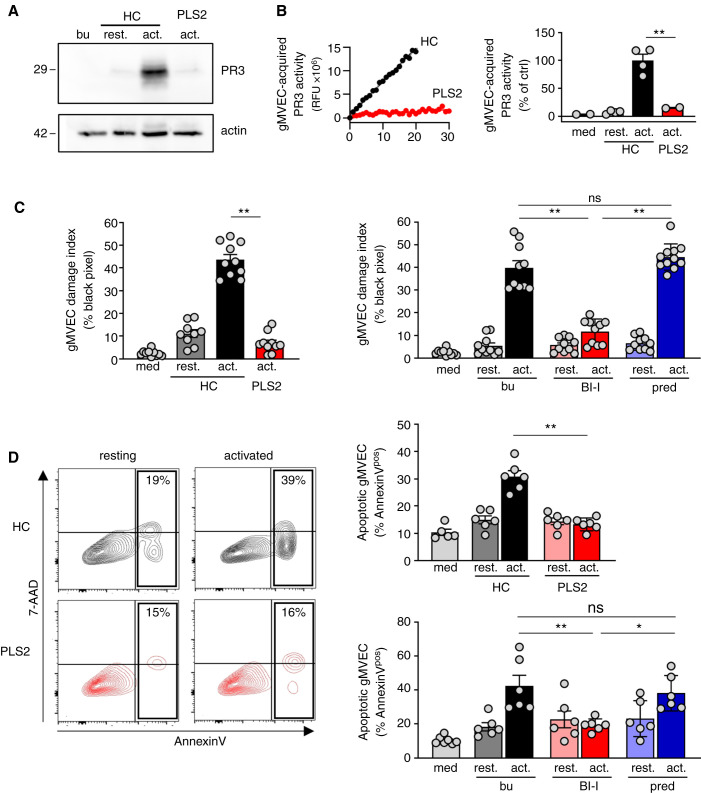
Figure 4.
Healthy control but not PLS neutrophils transfer proteolytically active PR3 to glomerular microvascular EC and both CatC gene deficiency and pharmacological CatC inhibition result in less neutrophil-induced glomerular microvascular EC injury. Confluent gMVEC monolayers were incubated with cf-SN from resting and activated HC or activated PLS neutrophils as indicated. After 1 hour, PR3 acquisition by glomerular microvascular EC was assessed by (A) immunoblotting using a PR3-specific mAb, and (B) FRET assay measuring proteolytic PR3 activity. Two independent experiments each with two HC and PLS2 neutrophils were performed. For assessing cell injury, glomerular microvascular EC monolayers were incubated with cf-SN from HC or PLS neutrophils, or from neutrophils that were differentiated from HSC in the presence of buffer, BI-I, or prednisolone as indicated. Each independent experiment includes neutrophils from two HC and PLS2, or two different HSC donors. Glomerular microvascular EC injury was determined (C) by phalloidin staining with the analysis of black pixel areas as in Figure 2D. The corresponding statistics is given (n=2), and (D) by Annexin V/7–AAD staining and flow cytometry (n=3). Contour plots of a typical experiment with cf-SN from resting and activated HC and PLS neutrophils are depicted together with the corresponding statistics of all experiments. *P<0.05 and **P<0.01.