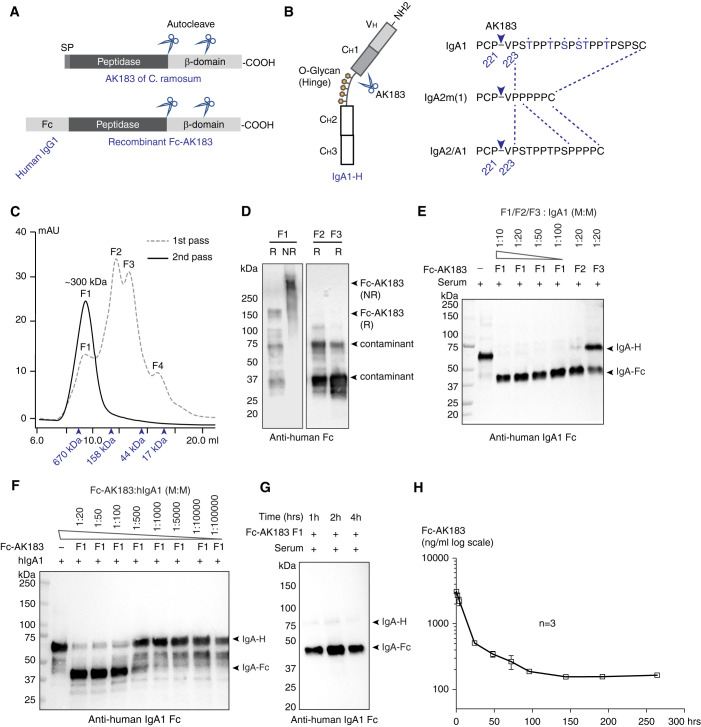
Figure 1.
Construction, production, and activity testing of recombinant Fc-AK183. (A) The domain architecture of C. ramosum AK183 IgA protease (top). In an N- to C-terminal order, the protein is composed of signal peptide (SP), metallopeptidase domain, and cell wall anchoring β domain. Multiple autoproteolytic cleavage sites were identified within the β domain. Recombinant Fc-AK183 fusion protein was constructed by replacing a signal peptide of AK183 with an Fc segment derived from human IgG1 (bottom). (B) Human IgA1 heavy chain comprises a VH domain followed by CH1–3 domains (left), of which CH1 and CH2 are connected by a glycosylated hinge segment. AK183 specifically cleaves Pro221-Val222 peptide bond immediately upstream of the hinge sequence, which is present in all IgA isotypes (right). (C) Recombinant Fc-AK183 produced by E. coli was purified by using SEC (1st pass) after Ni-affinity chromatography. The first elution fraction (F1) of approximately 300 kDa was expected to be the dimeric form of Fc-AK183 via Fc-Fc dimerization. Molecular weight markers are indicated by arrowheads (from left to right: 670, 158, 44, and 17 kDa). Four distinct peaks (F1–F4) were separately collected. F1 was subsequently analyzed by a 2nd pass on SEC, which then formed a single peak of dimeric Fc-AK183. This repurified F1 fraction was considered Fc-AK183 that was subsequently used in all experiments. (D) Immunoblotting blotting analyses of F1, F2, and F3 on reducing (R) and nonreducing (NR) SDS-PAGE with anti-human IgG Fc antibody confirmed dimeric Fc-AK183 in F1 fraction. (E) Protease activities of elution fractions F1–F3 were evaluated using human IgA1 as substrate. Anti-IgA1-Fc antibody detected both intact and Fc-AK183-cleaved IgA1 heavy chain running at approximately 70 kDa (IgA-H) and approximately 37 kDa [IgA(Fc)], respectively. Dilution series of F1 fraction showed higher activity than F2 and F3 in terms of complete digestion of IgA1 at higher dilution (1:100 versus 1:20; W: weight). (F) F1 IgA protease activity was further confirmed by a broader dilution series of the fraction against purified IgA1 with the presence of a production fragment of IgA-Fc. (G) Fc-AK183 cleaved all IgA1 in whole human serum within 1 hour. (H) After iv injection in mice (n=3), serum levels of Fc-AK183 remained detectable beyond 10 days (over 250 hours). The blood t1/2 of recombinant Fc-AK183 was calculated as 62.5 hours based on two-phase decay model (see Methods section).