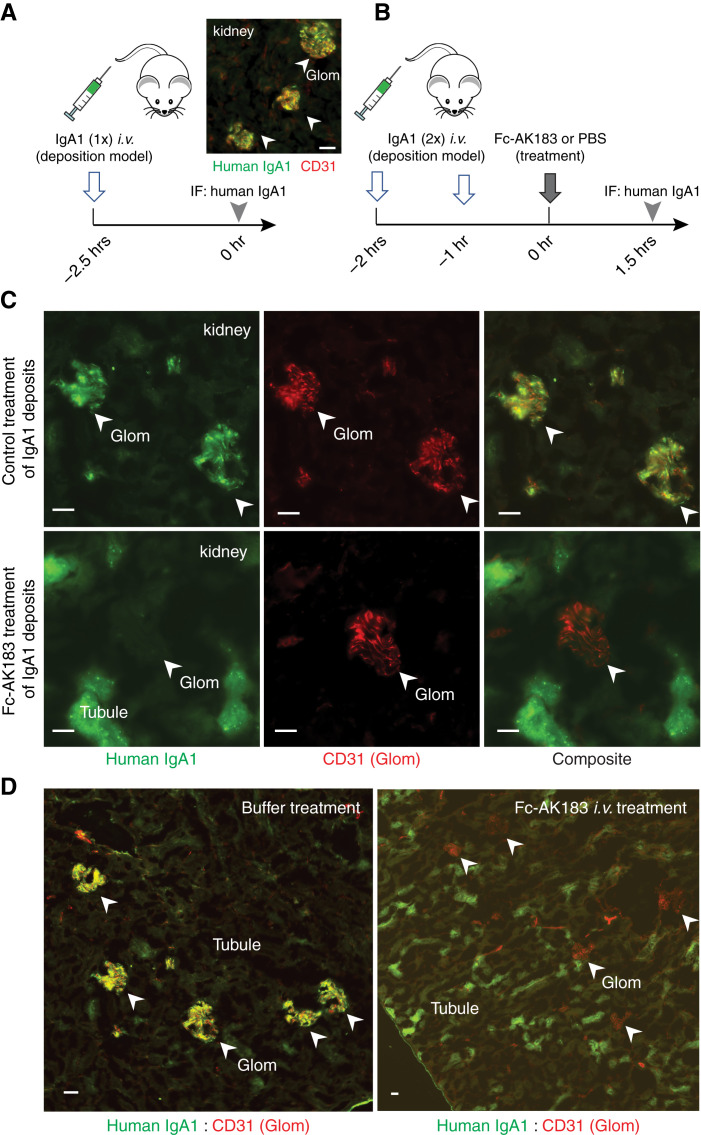
Figure 4.
Efficacy and kinetics of glomerular clearance of IgA1 deposits after bolus Fc-AK183 injection. (A) A passive IgA deposits model was created by bolus injection of purified human IgA1 in mice (left). IgA1 deposition in the glomerular mesangium was evident (right). (B) We followed a workflow to demonstrate the efficacy of therapeutic intervention by Fc-AK183. Wild-type BALB/c mice received two consecutive doses of purified human IgA1 injections. One hour after the second dose of IgA1, the mice received a treatment dose of either 5 mg/kg body wt Fc-AK183 or PBS control, and kidneys were harvested 1.5 hours later. (C) The buffer-treated group showed extensive IgA1 deposition in the glomerulus (marked with CD31 counterstain in red) (top). In contrast, mice receiving a treatment dose of 5 mg/kg Fc-AK183 completely lacked glomerular deposits. Instead, Fc-AK183 treatment resulted in IgA1 signals in proximal tubules (bottom). (D) Additional examples of contrasting differences between PBS and Fc-AK183 treatments on IgA1 renal deposition: Fc-AK183 completely removed the glomerular deposits of IgA1 and showed broad presence of tubular IgA1 signals, indicating renal clearance of IgA1. Scale bar, 30 μm.