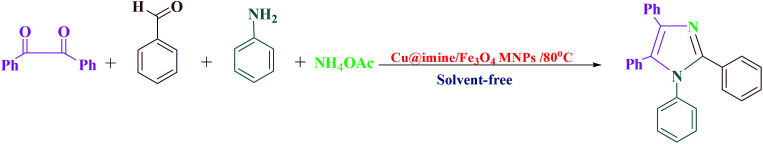
Optimization of the four-component reaction of benzil, benzaldehyde, benzylamine and ammonium acetate under various conditionsa.
| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalyst (mol%) | Temp. | Time (min) | Yieldb (%) |
| 1 | Ethanol | 0.36 | Reflux | 55 | 69 |
| 2 | Acetonitrile | 0.36 | Reflux | 55 | 61 |
| 3 | Water | 0.36 | Reflux | 80 | 78 |
| 4 | Dichloromethane | 0.36 | Reflux | 70 | 45 |
| 5 | Chloroform | 0.36 | Reflux | 70 | 49 |
| 6 | Tetrahydrofuran | 0.36 | Reflux | 70 | 43 |
| 7 | Solvent-free | 0.36 | 80 °C | 35 | 95 |
| 8 | Solvent-free | 0.24 | 80 °C | 55 | 79 |
| 9 | Solvent-free | 0.48 | 80 °C | 35 | 92 |
| 10 | Solvent-free | 0.36 | 25 °C | 120 | 35 |
| 11 | Solvent-free | 0.36 | 60 °C | 65 | 69 |
| 12 | Solvent-free | 0.36 | 70 °C | 45 | 75 |
| 13 | Solvent-free | 0.36 | 90 °C | 35 | 93 |
| 14 | Solvent-free | 0.36 | 100 °C | 35 | 91 |
| 15w | Solvent-free | BF3–SiO2/0.36 | 80 °C | 150 | 68 |
| 16 | Solvent-free | MgCl2/0.36 | 80 °C | 80 | 56 |
| 17 | Solvent-free | SbCl5–SiO2/0.36 | 80 °C | 150 | 65 |
Reaction conditions: benzil (1 mmol), benzaldehyde (1 mmol), benzylamine (1 mmol), ammonium acetate (4 mmol), and the required amount of catalyst.
The yields refer to the separated product.
