Figure 4. Glutamine metabolism impacts the anticancer immune response.
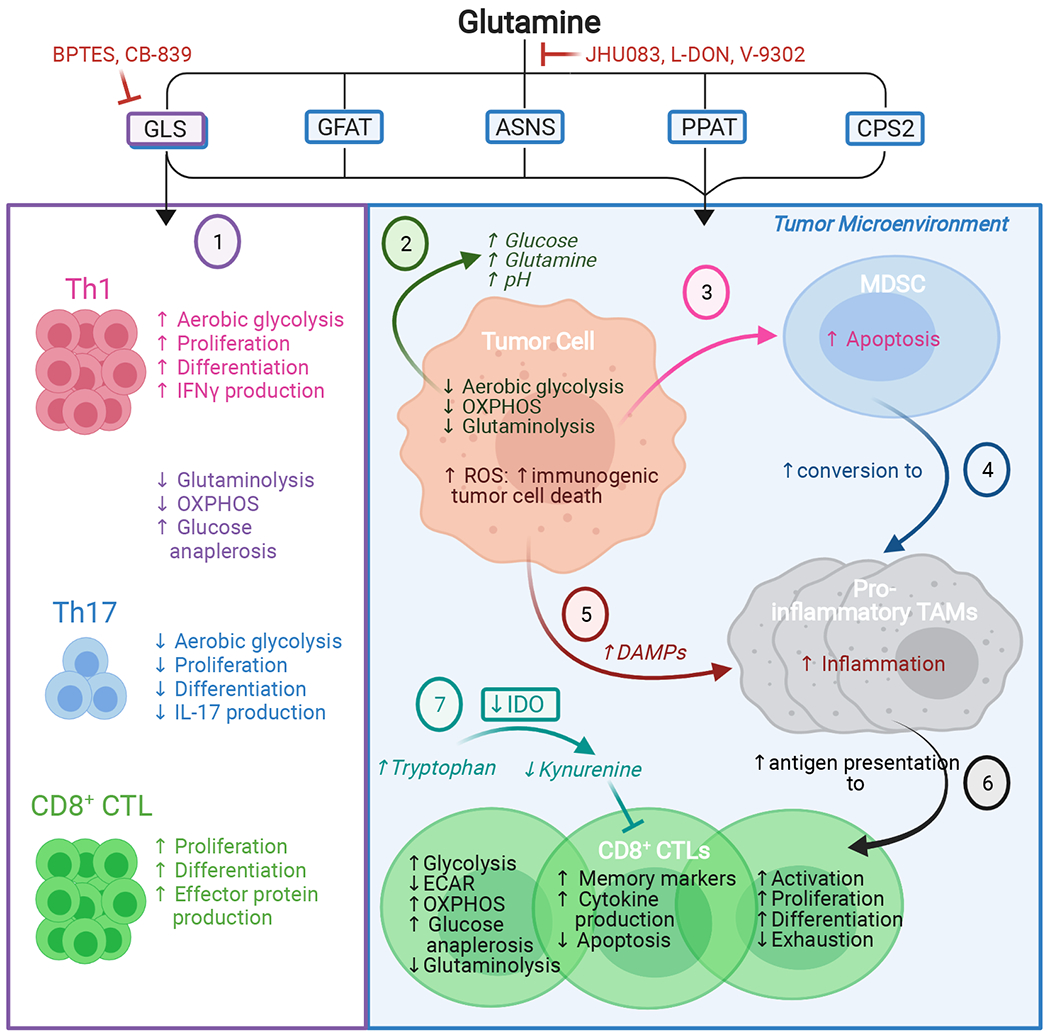
Graphical representation of the effects of allosteric GLS inhibitors (1) and non-selective glutamine antagonists (2-7). Selective GLS inhibition has distinct effects on the differentiation of CD4+ and CD8+ immune cell subsets, favoring CD4+ Th1 and CD8+ CTLs but suppressing CD4+ Th17 differentiation (1). Broader-spectrum glutamine antagonism alters the nutrient composition of the TME (2) and blocks MDSC generation and recruitment (3). Instead, glutamine antagonism favors conversion of MDSCs to pro-inflammatory TAMs, which inhibit tumor growth (4) and further activates pro-inflammatory TAMs via tumor cell-generated DAMPs (5). Inhibiting glutamine metabolism also directly modulates CD8+ CTL metabolism to promote a long-lasting, activated, memory-like phenotype; enhances antigen presentation by pro-inflammatory TAMs to CD8+ CTLs (6); decreases production of the immunosuppressive metabolite kynurenine in the TME (7); and increases CD8+ CTL tumor infiltration. These cumulative effects promote the anticancer immune response.
Abbreviations: ASNS, asparagine synthetase; BPTES, bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide; CPS2, carbamoyl phosphate synthetase II; CTL, cytotoxic T lymphocyte; DAMPs, damage/danger-associated molecular patterns; ECAR, extracellular acidification rate; GFAT, glutamine-fructose-6-phosphate transaminase; GLS, glutaminase; IDO, indoleamine-2,3-dioxygenase; IFNγ, interferon gamma; IL-17, interleukin 17; L-DON, 6-diazo-5-oxo-L-norleucine; MDSC, myeloid-derived suppressor cell; OXPHOS, oxidative phosphorylation; PPAT, phosphoribosyl pyrophosphate amidotransferase; TAMs, tumor-associated macrophages; Th1, T-helper 1; Th17, T-helper 17.
