Conformational dependency of solubility and heat of fusion of V(acac)3 in [Tea][BF4] and [C4mim][NTf2] ionic liquids. The melting temperature for all cases is 460 K.
| Ionic liquids | [Tea][BF4] | [C4mim][NTf2] | |||||
|---|---|---|---|---|---|---|---|
| Heat of fusion (ΔHf) | 1/γ∞ | 7.17 (kcal mol−1) | 5.68 (kcal mol−1) | 1/γ∞ | 7.17 (kcal mol−1) | 5.68 (kcal mol−1) | |
| Geometry | Solubility | Solubility | Solubility | Solubility | |||
| Conf1 | 0.287 | 1.30 × 10−2 | 2.63 × 10−2 | 1.83 | 2.41 × 10−2 | 5.70 × 10−2 | |
| Conf2 | 0.269 | 1.25 × 10−2 | 2.45 × 10−2 | 1.44 | 1.93 × 10−2 | 4.65 × 10−2 | |
| Conf3 | 0.277 | 1.29 × 10−2 | 2.54 × 10−2 | 2.06 | 2.71 × 10−2 | 6.34 × 10−2 | |
| Conf4 | 0.277 | 1.29 × 10−2 | 2.54 × 10−2 | 2.11 | 2.76 × 10−2 | 6.45 × 10−2 | |
| Conf5 | 0.297 | 1.38 × 10−2 | 2.72 × 10−2 | 1.92 | 2.53 × 10−2 | 5.95 × 10−2 | |
| x̄ | 0.281 | 1.30 × 10−2 | 2.58 × 10−2 | 1.87 | 2.47 × 10−2 | 5.82 × 10−2 | |
| σ E | 0.008 | 0.00041 | 0.00074 | 0.024 | 0.0003 | 0.00067 | |
| δ E | 0.003 | 0.0002 | 0.0003 | 0.011 | 0.00014 | 0.00030 | |
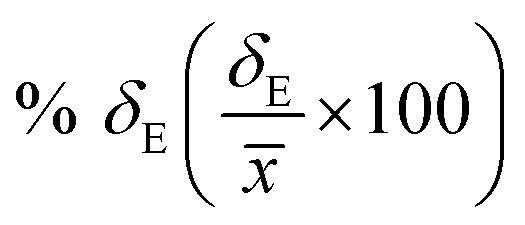
|
1.06 | 1.54 | 1.16 | 0.59 | 0.57 | 0.52 | |
