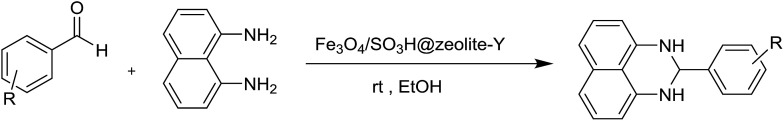
Synthesis of dihydroperimidine catalyzed by Fe3O4/SO3H@zeolite-Ya.
| |||||
|---|---|---|---|---|---|
| Entry | R | Product | Time (min) | Yieldb (%) | mprep/mplit. (°C) |
| 15 | H | 6a | 4 | 98 | 104–106 (104–106)20 |
| 16 | 2-NO2 | 6b | 4 | 98 | 180–192 (192–194)20 |
| 17 | 3-NO2 | 6c | 4 | 90 | 200–202(183–185)39 |
| 18 | 4-NO2 | 6d | 3 | 98 | 224–226 (201–202)20 |
| 19 | 4-OMe | 6e | 4 | 90 | 160–162 (161–163)20 |
| 20 | 3,4-OMe | 6f | 5 | 89 | 214–216 (205–207)20 |
| 21 | 2-OH | 6g | 5 | 90 | 180–182 (192–193)40 |
| 22 | 2-OH, 5-Br | 6h | 4 | 95 | 148–150 (165–166)20 |
| 23 | 2-OH, 4-OMe | 6i | 6 | 89 | 204–206 (168–169)20 |
| 24 | 3-Cl | 6j | 4 | 95 | 144–146 (145–146)20 |
| 25 | 4-Cl | 6k | 4 | 98 | 171–173 (173–174)20 |
| 26 | 2,3-Cl | 6l | 4 | 95 | 130–132 (131–133)21b |
| 27 | 5-NO2c | 6m | 6 | 88 | 340–342 (350)20 |
Reaction conditions: aldehyde (1 mmol), 1,8-diaminonaphthalene (1 mmol), Fe3O4/SO3H@zeolite-Y (0.008 g), in EtOH at 25 °C.
Isolated yield.
5-Nitrofuran-2-yl.
