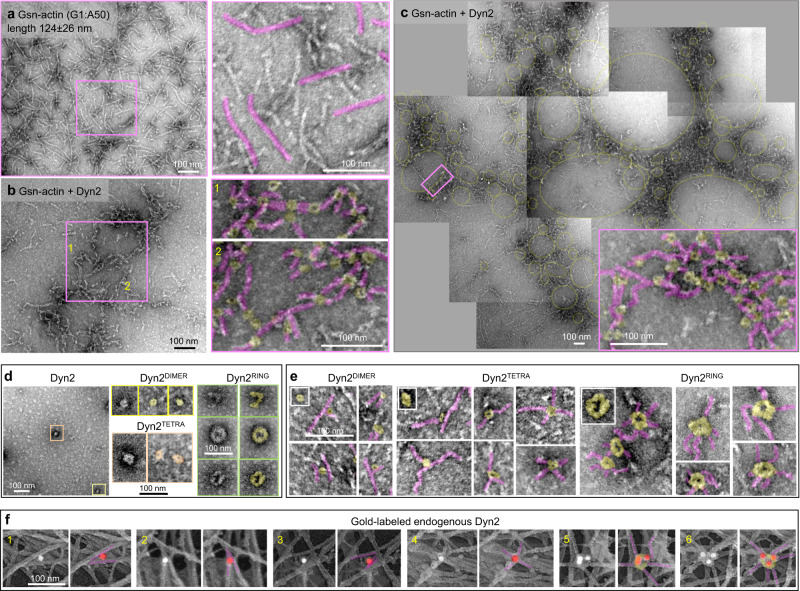
Fig. 2. Dynamin cross-links actin filaments into branched networks.
a–c Representative TEM images of branched actin networks assembled by gelsolin-capped short actin filaments (Gsn-actin) and Dyn2. Gsn-actin (polymerized at the ratio of G1:A50) is shown in (a), and the actin networks assembled in the presence of Dyn2 are shown in (b). Several electron micrographs were stitched together to show the global organization of actin networks formed by Dyn2 in (c). A higher magnification image of the boxed region shows the structural arrangement of actin filaments (pink) and Dyn2 (yellow) within the networks. d Representative micrographs of negatively stained recombinant Dyn2. Boxed higher magnification images show distinct oligomerization states of dynamin: dimeric (yellow), tetrameric (orange), partial/full ring (green). e High magnification images of actin filaments (pink) associated with distinct Dyn2 oligomerization forms (yellow). Insets show images of only Dyn2 at different oligomerization states. f Immunogold platinum replica electron micrographs focusing on actomyosin cortex in MDCK cells. Cellular localization of endogenous Dyn2 was determined by monoclonal Dyn2 antibody and gold-conjugated secondary antibody (white particles). To better visualize the gold particles within tightly packed actin networks, white densities associated with gold particles were pseudo-colored red, and the actin filaments associated with gold particles were pseudo-colored pink. The presence of multiple gold particles identifies the formation of macromolecular dynamin complexes on actin filaments, consistent with the formation of dynamin rings. Representative images of two independent experiments.