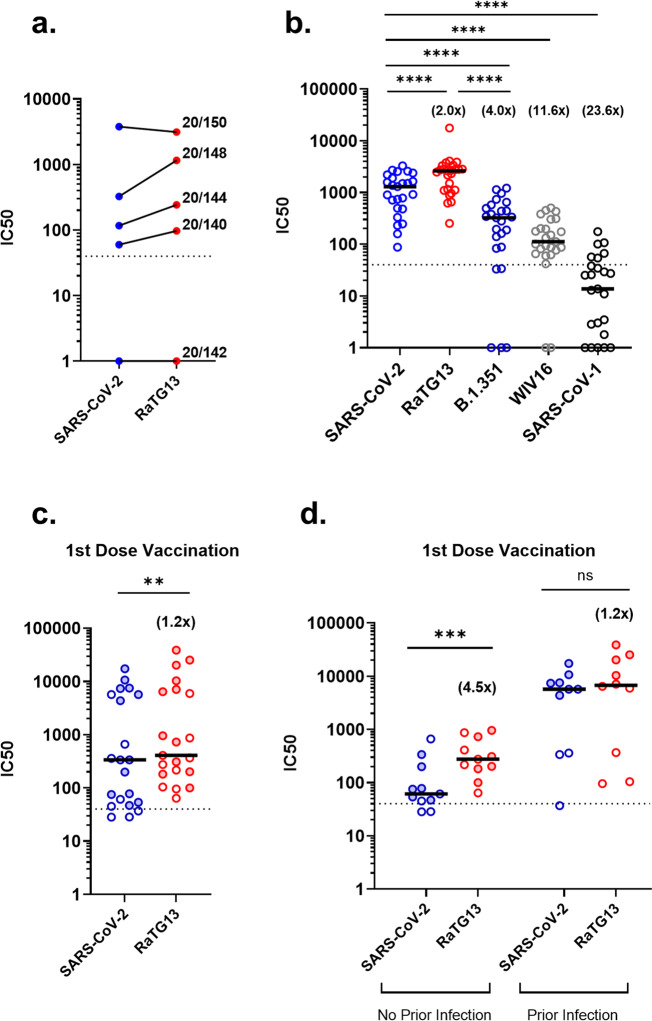
Fig. 1. Differences in neutralisation titres between SARS-CoV-2 and RaTG13 by pMN Assay.
a Comparison of neutralisation titres between SARS-CoV-2 and RaTG13 using the WHO International Reference Panel for anti-SARS-CoV-2 immunoglobulin. Three of the four sera showed increased neutralisation titres against RaTG13. b Comparison of neutralisation titres between SARS-CoV-2 (n = 25), RaTG13 (n = 25, p = <0.0001), B.1.351 (Beta) (n = 25, p = <0.0001) variant of concern, SARS-CoV-1 (n = 25, p = <0.0001) and WIV16 (n = 25, p = <0.0001) using convalescent sera derived from patients and healthcare workers. c Comparison of neutralisation titres between SARS-CoV-2 and RaTG13 against sera from single-dose vaccinated healthcare workers (n = 21, p = 0.0016). d Differences in neutralisation titre from single dose vaccinated healthcare workers split by ‘no prior infection’ (n = 11, p = 0.001) or ‘prior infection’ (n = 10, p = 0.084) with SARS-CoV-2. Full circles denote healthcare workers vaccinated with BNT162b2 (n = 12), whereas open circles denote vaccination with AZD1222 (n = 9). Numbers in brackets denote fold changes relative to SARS-CoV-2. Wilcoxon matched pairs signed rank tests were used in panels (b), (c) and (d). Dotted lines in graphs denote the assay’s lower limit of detection. IC50 was calculated by fitting a non-linear regression curve using Graphpad Prism 8 software. All n values constitute of biologically independent samples.