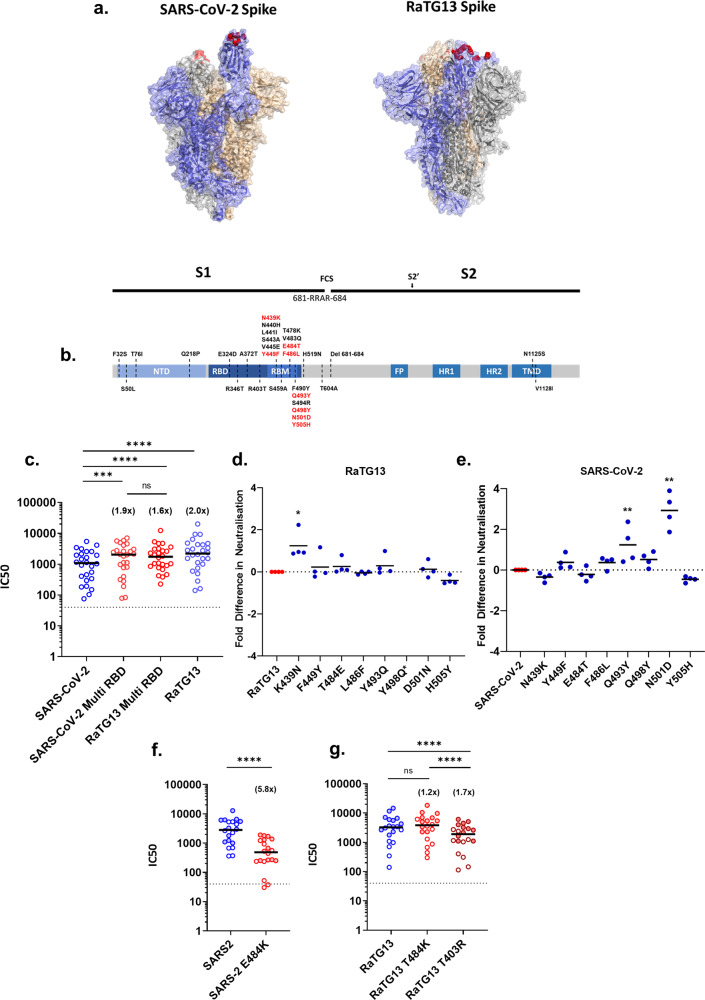
Fig. 2. Key amino acid residues affect antibody neutralisation against SARS-CoV-2 and RaTG13.
a Structures of SARS-CoV-2 (PDB: 6X2A) and RaTG13 (PDB: 7CN4). The highlighted (red) amino acids denote the ACE2 contact residues that were substituted to generate the Multi RBD plasmids for subsequent pMN assays. b Simplified schematic highlighting the functional domains within the SARS-CoV-2 Spike protein. The amino acid substitutions found in RaTG13 have been labelled across the diagram, with red text denoting the substitutions displayed in panel (a), which were built into chimeric Spikes. (FCS, furin cleavage site; NTD, N-terminal domain; RBD, receptor binding domain; RBM, receptor binding motif; FP, fusion peptide; HR, heptad repeat; TM, transmembrane domain). (c) Repeated experiments showing the neutralisation titres, by pMN assay, of SARS-CoV-2 (n = 25), RaTG13 (n = 25, p = <0.0001) and both Sars-CoV-2 Multi RBD (n = 25, p = 0.0005) and RaTG13 Multi RBD (n = 25, p = 0.0043) mutants with the same set of convalescent sera used in Fig. 1. We did not observe a significant difference between the Multi RBD results (n = 25, p = 0.9007). d, e Using a set of four convalescent sera derived from patients, each pseudotype mutant was assayed by pMN and IC50s were then converted to fold changes against their original RaTG13 (d) (K439N; n = 4, p = 0.0194) or SARS-CoV-2 (e) (Q439Y; n = 4, p = 0.0088, N501D; n = 4, p = 0.0069) background. *RaTG13 Y498Q was not performed due to low PV titre (Supplementary Fig 3). f Lysine substitution at position 484 in SARS-CoV-2 Spike significantly reduced neutralisation (n = 20, p = <0.0001). g Lysine substitution at position 484 in RaTG13 however showed a subtle, non-significant increase in neutralisation titres (n = 20, p = 0.1054), whereas an arginine substitution at position 403 significantly reduced the neutralisation titres compared to RaTG13 (n = 20, p < 0.0001) and RaTG13 T484K (n = 20, p < 0.0001). Comparisons of neutralisation in panel (f) and (g) were made using sera from doubly vaccinated HCWs (n = 20). Full circles denote healthcare workers vaccinated with BNT162b2 (n = 11), whereas open circles denote vaccination with AZD1222 (n = 9). Numbers in brackets denote fold changes. Wilcoxon matched pairs signed rank tests was used in panel (c), (f) and (g). Student’s test was used in panels (d) and (e). IC50 was calculated by fitting a non-linear regression curve using Graphpad Prism 8 software. All n values constitute biologically independent samples.