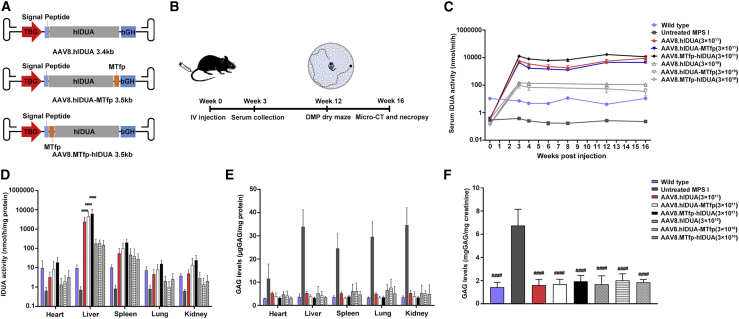
Figure 2.
Biochemical correction in peripheral tissues of treated mice
(A) Schematic of the AAV8.hIDUA, AAV8.hIDUA-MTfp, and AAV8.MTfp-hIDUA vectors. The AAV vectors contain the TBG promotor, a Kozak sequence, the signal peptide, the unmodified hIDUA or modified hIDUA (hIDUA-MTfp or MTfp-hIDUA) transgenes, and the bGH polyadenylation sequence flanked by AAV2 inverted terminal repeats (ITRs). (B) A summary of the in vivo experiments. (C) Serum IDUA activity from weeks 0–16 after injection was measured. Mean ± SEM are shown. IDUA activity in all high-dose-treated mice is significantly higher (p < 0.05) than levels found in wild-type mice from weeks 3–16. (D) IDUA activity in peripheral tissues was detected 16 weeks after injection. ####p < 0.0001 compared with the wild-type group. Mean ± SD are shown. (E) GAG levels in peripheral tissues were detected 16 weeks after injection. GAG levels in all groups are significantly lower (p < 0.001) than levels found in untreated MPS I mice. Mean ± SD are shown. (F) Urine GAGs were detected 16 weeks after injection. Mean ± SEM are shown. ####p < 0.0001 compared with the untreated MPS I group. (C–F) Wild-type mice, n = 7. Untreated MPS I mice, n = 7. 3 × 1011 GC/mouse dose group: AAV8.hIDUA-treated mice, n = 8; AAV8.hIDUA-MTfp-treated mice, n = 9; AAV8.MTfp-hIDUA-treated mice, n = 9. 3 × 1010 GC/mouse dose group, n = 7.