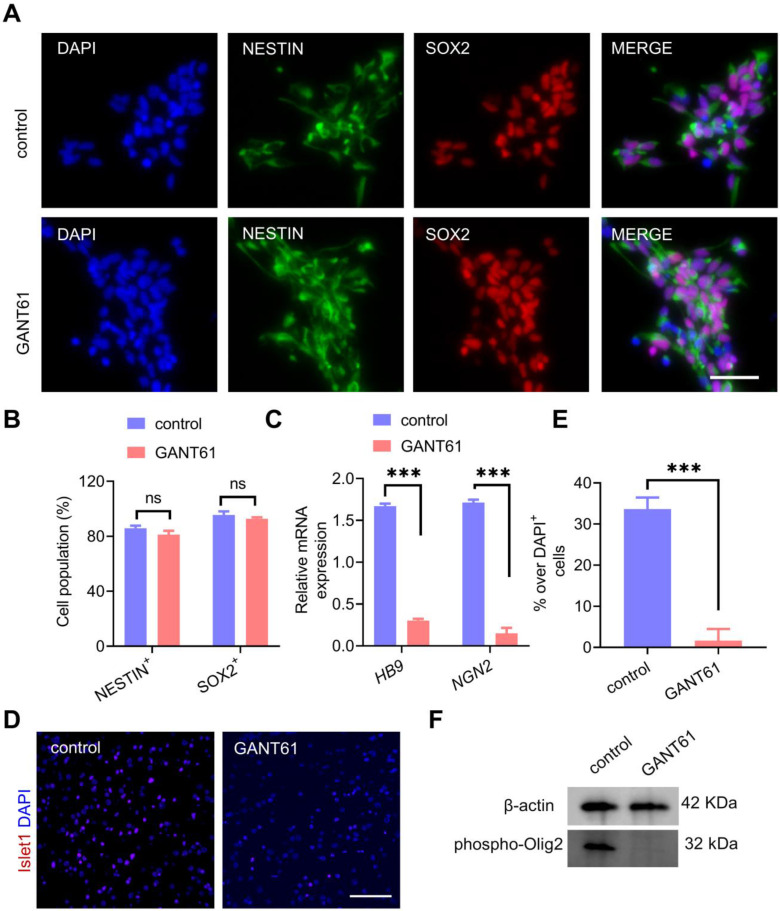
Figure 2.
Shh inhibition abolished MN formation and facilitated the MN-OL fate switch by blocking Olig2 phosphorylation at Ser147. A Representative immunofluorescent staining images of control NPCs and GANT61-NPCs showed coexpression of NPC markers (NESTIN and PAX6) at d0 of differentiation; scale bar, 50 μm. B The corresponding quantification of NESTIN+/PAX6+ NPCs from control NPCs and GANT61-NPCs (at d0 of differentiation, ns p > 0.05, a two-tailed Student's t test). GANT61 treatment did not affect NPC formation from hiPSCs. C The mRNA expression levels of MN marker genes (HB9 and NGN2) were analyzed by qPCR in control NPCs and GANT61 NPCs (at d0 of differentiation, *** p < 0.001, by a two-tailed Student's t test). GANT61 treatment downregulated MN-specific marker genes (HB9 and NGN2). D Representative immunofluorescent staining images of the MN marker Islet1 in control NPCs and GANT61-NPCs; scale bar, 100 μm. E Quantification of the expression of Islet1 in the control NPCs and GANT61 NPCs. GANT61 treatment downregulated the MN-specific protein marker Islet1 (*** p < 0.001, by a two-tailed Student's t test). F Western blot analysis of Olig2 phosphorylation at Ser147. GANT61 treatment blocked Olig2 phosphorylation at Ser147. Control-NPCs and GANT61-NPCs were immunoblotted with an anti-Olig2 (phospho-S147) antibody, and proteins were normalized to the housekeeping gene β-actin. The graphs represent the individual data points and the mean ± SEM of three independent experiments. Immunofluorescence images are representative of n = 3 biological replicates.