Abstract
Purpose of Review
COVID-19 is now a global pandemic and the illness affects multiple organ systems, including the cardiovascular system. Long-term cardiovascular consequences of COVID-19 are not yet fully characterized. This review seeks to consolidate available data on long-term cardiovascular complications of COVID-19 infection.
Recent Findings
Acute cardiovascular complications of COVID-19 infection include myocarditis, pericarditis, acute coronary syndrome, heart failure, pulmonary hypertension, right ventricular dysfunction, and arrhythmia. Long-term follow-up shows increased incidence of arrhythmia, heart failure, acute coronary syndrome, right ventricular dysfunction, myocardial fibrosis, hypertension, and diabetes mellitus. There is increased mortality in COVID-19 patients after hospital discharge, and initial myocardial injury is associated with increased mortality.
Summary
Emerging data demonstrates increased incidence of cardiovascular illness and structural changes in recovered COVID-19 patients. Future research will be important in understanding the clinical significance of these structural abnormalities, and to determine the effect of vaccines on preventing long-term cardiovascular complications.
Keywords: COVID-19, Cardiovascular complications, Post-acute COVID complications, Long COVID
Introduction
Coronaviruses are common pathogens that circulate in the human population where they typically cause a mild respiratory illness [1]. In contrast, the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle Eastern acute respiratory syndrome coronavirus (MERS-CoV) were initially contracted via animal to human transmission and are responsible for severe respiratory disease in some affected individuals. The severe acute respiratory syndrome (SARS) pandemic began in 2002 in China and was believed to be contracted initially via bat to human transmission. This pandemic was controlled with aggressive case detection and isolation measures, and no case of SARS has been detected since 2004 [2]. Middle Eastern respiratory syndrome (MERS) was first noted in Saudi Arabia in 2012 and can have up to a 35% mortality rate. Camels are believed to be the animal reservoir and thus represent an ongoing risk of repeat outbreak [3].
In December 2019, a new severe viral pneumonia was discovered in Wuhan city of the Hubei province of China. The coronavirus disease 2019 (COVID-19) is a respiratory viral illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 is now a global pandemic, and at the time of this writing has infected more than 340 million individuals and caused more than 5,500,000 deaths worldwide [4]. Despite being defined as a respiratory virus, it has been shown to cause significant multiorgan dysfunction in the acute setting including acute respiratory distress syndrome, renal injury, liver injury, myocardial injury, and systemic shock [5–6].
Data are now emerging demonstrating ongoing organ dysfunction in the post-infectious period with increasing attention being granted towards reported symptoms well after the acute phase of illness [7•, 8]. The clinical entity of post-acute COVID syndrome (PACS) or the colloquial “long COVID” has been proposed to describe an assortment of multiorgan symptoms which arise or persist beyond 4–12 weeks post-infection. To date, the question of whether these symptoms are directly related to a complication from the acute illness versus an alternative pathologic process has not been fully elucidated [9, 10]. Despite this uncertainty, however, PACS has clearly emerged as a substantial cause of subacute to chronic morbidity, with 57% of patients hospitalized for COVID-19 reporting ongoing symptoms 1 year after initial COVID-19 infection. The most frequently described symptoms include fatigue, muscle weakness, headache, sleep difficulties, and breathlessness among others [11•].
In this review, we seek to consolidate emerging data regarding the long-term cardiovascular consequences of COVID-19 infection, and anticipate future investigative needs based on existing data regarding cardiovascular complications following viral illnesses.
Acute Cardiovascular Complications of COVID-19
Acute cardiovascular complications of SARS-CoV-2 infection are well-described, with the most common being myocardial injury, arrhythmia, heart failure, and acute coronary syndrome (ACS). These acute complications can lead to longer-term sequelae that may require chronic cardiovascular care. It should be noted that these data largely reflect cardiovascular complications described prior to broad vaccine availability, with further investigation warranted now that vaccines are more widely available.
Arrhythmia has been noted in up to 10.4% of COVID-19 patients with moderate to severe COVID-19 disease, with the most frequently observed being atrial fibrillation, non-sustained ventricular tachycardia, and bradyarrhythmias [12–14]. Rates of heart failure complicating the course of COVID-19 illness have ranged from 2.8% in COVID-19 inpatients of all levels of care to 24% of patients who died from the disease [15–17]. Myocardial infarction has been noted in up to 1.3% of all COVID-19 patients and up to 4.9% of patients who died from the illness [18–20].
Myocarditis has been observed in 0.15% of patients diagnosed with COVID-19, a phenomenon which contributed to a 42% increase in myocarditis incidence throughout the USA during the pandemic study period compared to the year prior [21]. It should be noted, however, that this figure is based on ICD-10 coding for the diagnosis of myocarditis. Myocarditis due to COVID-19 infection has been difficult to demonstrate, as there is a general resistance to the diagnostic gold standard of endomyocardial biopsy due to its invasive nature and limited sensitivity, and isolation protocols for COVID-19 patients made this even less likely to occur. Presence of SARS-CoV-2 has of yet only been demonstrated in the macrophages of cardiomyocytes in a patient suffering from presumed myocarditis and was associated with mild inflammation without overt necrosis [22]. An autopsy case series showed 61.5% of patients demonstrated a meaningful viral load of SARS-CoV-2 in their myocardium, though this was not associated with inflammation [23]. In all, this suggests that true virally induced myonecrosis is a rare entity. As a result, diagnosis of myocarditis in COVID-19 patients could reasonably be considered suspected based on elevated cardiac biomarkers and other clinical indications, but the true prevalence is unknown and likely rare. Acute pericarditis in the setting of COVID-19 infection is fairly rare and is frequently seen in conjunction with myocardial inflammation. Diagnosis is generally made via electrocardiogram findings of diffuse ST segment elevation and PR depression but can also present with pericardial effusion and tamponade in the minority of cases [24]. This has to date only been reported in case series, so overall prevalence is not known.
Myocardial injury has been identified in up to 21% of COVID-19 patients in the acute setting and plays a valuable prognostic role, with elevated cardiac biomarkers predictive of intensive care unit (ICU) admission, mechanical ventilation, arrhythmias, and right ventricular dysfunction [25–28]. Myocardial injury has also been associated with increased in-hospital mortality. In one cohort study of hospitalized COVID-19 patients, myocardial injury was observed in 20% and was in turn associated with an in-hospital mortality rate of 51% in comparison to a rate of 4.5% in those hospitalized without evidence of myocardial injury [26]. Presence of myocardial injury during index hospitalization is also associated with development of post-acute sequelae from COVID-19 illness in the months after acute illness [29•].
Studies employing echocardiographic assessment in the setting of acute COVID-19 have shed further light on potential mechanisms of longer-term cardiovascular effects. Pulmonary hypertension and right ventricular dysfunction have been observed in up to 12% and 14–33%, respectively, of patients admitted for COVID-19. Both findings are observed more frequently in those with prior cardiac comorbidities and have been associated with more severe lung disease and higher in-hospital mortality rates [30–32]. Though described, the incidence of left ventricular dysfunction is not entirely clear. Dweck et al. showed that in all COVID-19 positive patients screened, left ventricular dysfunction was present in 39%. This involved a cohort in which 46% of patients had no pre-existing cardiac disease [31]. Other studies have demonstrated lower incidence of left ventricular dysfunction, with most patients exhibiting preserved to hyperdynamic left ventricular systolic function [32–33].
Long-Term Cardiovascular Complications of COVID-19
Given the high prevalence of cardiac complications in the acute phase of infection and a burgeoning literature base describing residual symptoms and organ dysfunction in post-acute COVID-19 patients, further attention is being granted towards long-term cardiovascular complications of COVID-19 infection. It is not yet clear to what extent these complications are secondary to unique pathologic processes rather than direct consequences of acute infectious complications.
Emerging long-term outcomes data demonstrate a significant burden of cardiovascular disease following acute infection with COVID-19. A large analysis from the US Department of Veterans Affairs (VA) databases recently demonstrated an excess burden of cerebrovascular disorders, arrhythmia, ischemic heart disease, heart failure, and thrombotic disorders between 30 days and 12 months after COVID-19 infection in comparison to control cohorts [34••]. In total, COVID-19 infection was associated with a greater than 50% increase in risk of one of these complications over the stated time frame (HR 1.63, 95% CI 1.59–1.68). Strikingly, these observations were made even in those who were not hospitalized for acute infection, though the risk rose in a graded fashion based on the highest level of care required during the initial phase of infection with the greatest burden occurring in those requiring intensive care. Of all complications investigated in this study, atrial fibrillation and heart failure accounted for the greatest excess burden, with both occurring in more than 10 additional individuals per 1,000 persons that were more than 30 days out from an acute COVID-19 infection.
Additional published studies both corroborate and expand upon the findings from the VA database. New-onset diabetes and major adverse cardiac events (MACE) have also been demonstrated to occur significantly more often in post-COVID patients than in matched controls [7•]. One investigation of individuals recovered from acute COVID-19 infection revealed that at 1 year follow-up, new-onset hypertension and de novo heart failure were present in 2% of patients, and there was an increased need for readmission for heart failure medication augmentation [11•]. Additionally, 2.7% of patients in this same study developed new right-sided heart failure in the absence of hypertension or left heart failure. Given the findings of frequently abnormal echocardiograms in the acute setting, however, it is difficult to know if this represents structural change in the post-infectious setting or dysfunction that was simply not assessed during the index admission. While pulmonary hypertension is often seen in the acute phase of the illness, this has not been frequently seen on follow-up echocardiography [33].
A recent prospective study demonstrated that presence of myocardial injury assessed via elevated high-sensitivity troponin during an index hospitalization for COVID-19 was associated with ongoing symptoms at 12 months as well as increased hospital readmission rates and mortality [29]. Other groups have also reported a substantial mortality rate in the year following COVID-19 infection with rates ranging from 1.3 to 12% [7•, 11•, 35]. These events are often attributed to sudden cardiac death felt to be related to arrhythmia or thrombosis.
Characterization of post-COVID-19 structural changes in the myocardium are forthcoming. Medium-term follow-up of 2–3 months has shown a staggering 78% of patients recovered from COVID-19 infection with abnormalities on cardiac magnetic resonance imaging (CMR), with 60% of them showing evidence of inflammation based on elevated native T1 and T2 enhancement. Focal scarring and pericardial enhancement were also present [36•]. Huang et al. discovered that patients recovered from COVID-19 infection who suffered from ongoing cardiac symptoms demonstrated myocardial edema and late gadolinium enhancement (LGE) in 54% and 38% of patients, respectively, on CMR [37]. LGE was primarily located in the interventricular septum, anterior, anterolateral, and inferior walls of the left ventricle. They also found that abnormal CMR findings correlated with decreased right ventricular (RV) function. Nuzzi et al. employed echocardiography to assess RV function after recovery from severe COVID, and while their data failed to reproduce evidence of overt RV dysfunction by tricuspid annular plane systolic excursion (TAPSE) or RV fractional shortening, it did show impaired RV longitudinal strain in 42% of patients studied [33]. These findings are suggestive of impaired RV systolic function that persists beyond the acute infectious phase, though the clinical significance and natural history of this finding remains to be seen.
Clues of Cardiovascular Complications from Other Viral Illnesses
In the absence of substantial primary data regarding long-term cardiovascular sequelae of COVID-19 infection, one can turn to existing data surrounding other viral infections for insights and guidance. Severe acute respiratory distress syndrome caused by the SARS-CoV in 2002–2003 was also a global health concern. Long-term follow-up of these patients revealed hyperlipidemia, abnormal glucose metabolism, and cerebrovascular accident (CVA) [38]. This appeared to be independent of steroid use. Patients recovering from pneumonia infection have been observed to have increased cardiovascular complications such as myocardial infarction, CVA, and fatal coronary disease in the 10 years following hospitalization [39]. Furthermore, vaccination against influenza has also demonstrated a reduced risk of MI and out of hospital cardiac arrest [40]. Together, these data suggest that post-infectious COVID-19 patients are likely at risk for increased cardiovascular events and metabolic abnormalities, which we have already started to observe. The mechanism for this predisposition towards atherosclerotic cardiovascular disease is not entirely clear but is likely related to the proinflammatory response generated by viral infection. It has long been established that inflammation plays a crucial role in development and destabilization of atherosclerotic plaque, and pivotal trials have demonstrated increased cardiovascular events in patients with evidence of chronic inflammation in the form of elevated C-reactive protein [41–42]. The inflammatory response causes macrophage migration to low-density lipoprotein (LDL) deposits in the subintimal space of an artery, where they phagocytose cholesterol deposits and become foam cells. They then undergo apoptosis, whereby a subintimal necrotic core propagates. Cytokines also play an important role in thinning the fibrous cap of these atherosclerotic plaques via matrix metalloproteases. Thus, the inflammatory response causes both expansion of the atherosclerotic plaque and thinning of the stabilizing cap, making it more vulnerable to rupture [43]. Additionally, platelet activation is enhanced through multiple inflammation-mediated mechanisms [44]. Respiratory viral infections such as influenza and now COVID-19 trigger robust native inflammatory and prothrombotic responses, indicating a possible mechanism for the observed increase in cardiovascular events following viral infection [45–46].
Discussion
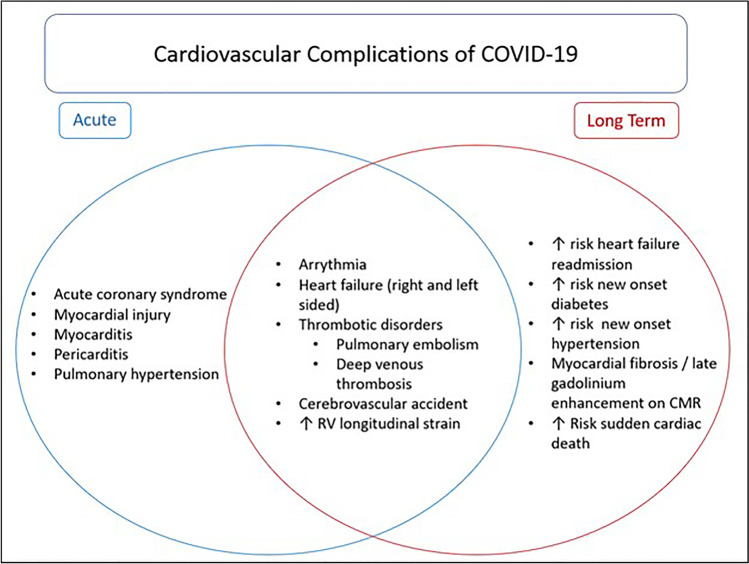
COVID-19 has drastically altered our way of life and causes significant morbidity and mortality in the acute setting. We are now discovering that many patients that suffer acute COVID infection continue to have significant cardiovascular complications in the post-acute setting as well (Fig. 1), adding to the already high burden of cardiovascular morbidity and throughout the USA and the rest of the world [47].
Fig. 1.
Acute and long-term cardiovascular complications associated with COVID-19 infection
Acute myocardial injury is observed in a significant number of patients acutely infected with SARS-CoV-2. Various mechanisms of injury have been proposed. Angiotensin I-converting enzyme 2 (ACE2) is a receptor expressed heavily on myocardial and alveolar surfaces and is the target of SARS-CoV-2’s spike protein to gain cellular entry [48]. This has been hypothesized as a mechanism for direct myocardial injury. Indeed, SARS-CoV-2 has been discovered in the myocardium via polymerase chain reaction (PCR) suggesting a pathogenicity via direct toxicity [23,49]. Alternatively, it is suggested that the inflammatory milieu caused by helper T cells and hypoxia during acute infection lead to a cytokine storm that can be cardiotoxic [35]. Lastly, patients with severe COVID-19 infections are older and have more underlying cardiovascular disease, so it would stand to reason that they would experience increased cardiovascular morbidity and mortality [50–51].
Acute cardiovascular complications include arrhythmia, myocarditis, acute coronary syndrome, pulmonary hypertension, and RV dysfunction. We are now beginning to understand how COVID-19 infection can impact long-term cardiovascular health. New-onset hypertension was noted in one study which could be due to disruption in the renin angiotensin system, wherein SARS-CoV-2 binds to ACE2 and promotes its degradation, thus promoting sodium and water retention [52]. This has been described in the setting of acute infection, but it is unclear why this would occur in the post-acute period. New-onset abnormal glucose metabolism and diabetes was seen in patients recovered from SARS-CoV infection as well [38]. It is unclear whether this is due to metabolic dysregulation because of the viral infection, or sequelae of the use of glucocorticoids as standard treatment for severe infection [53–54]. Increased incidence of myocardial infarction was also noted. While the cause of this is unclear, it has been well described following other acute respiratory illnesses such as pneumonia and chronic obstructive pulmonary disease exacerbation [11•, 39]. Thus, regular primary care screening for diabetes and hypertension should remain a priority, which can be challenging in the ever-changing pandemic healthcare landscape. Clinicians should remain vigilant as well for signs and symptoms of ACS in light of the increased prevalence of MACE following viral infection.
Acute PH and RV dysfunction in the setting of COVID-19 infection is postulated to result from altered pulmonary hemodynamics due to hypoxic vasoconstriction, use of positive end expiratory pressure in mechanical ventilation, pulmonary endothelial injury, and thrombotic or thromboembolic processes locally in the pulmonary vasculature [30]. Follow-up echocardiography in the post-acute setting failed to show overt RV dysfunction in the form of depressed TAPSE or RV fractional shortening, but has shown impaired RV longitudinal strain [33]. While impaired RV longitudinal strain is associated with increased mortality in the acute setting, the long-term consequences remain to be seen [55]. In left ventricular assessment, impaired longitudinal strain is a more sensitive marker for systolic dysfunction and is predictive of future heart failure [56–58]. Thus, the cardiovascular clinician would reasonably anticipate some incidence of right heart dysfunction and failure in the COVID-19-recovered population. The origin of impaired RV longitudinal strain is unclear, but could be related to myocardial inflammation, hypoxia, and ventilation-induced trauma, which are frequently present during the acute phase of the illness [59].
CMR has revealed edema and left ventricular LGE in a small group of patients with ongoing cardiovascular symptoms in the post-acute phase of their illness. This is favored to be a result of the myocardial inflammation suffered during the acute COVID-infection, though data to correlate with laboratory evidence of myocardial injury during index hospitalization are not available [37]. Given the increased mortality seen in the year after COVID-19 infection, this raises concern for possible arrhythmogenic or thrombotic causes of sudden death during the post-acute phase of COVID-19 illness.
Study Limitations
Certain limitations of this review and the data presented should be noted. Given the COVID-19 disease was first recognized just over 2 years ago, there is limited long-term data from which to draw conclusions. This information is just now being gathered and published, so the volume of studies from which to draw long-term conclusions currently remains modest. The data presented reflect short- and long-term complications of COVID-19 infection largely in unvaccinated populations. Therefore, complication rates in the acute setting and predisposition for post-acute sequelae may be different for vaccinated individuals. Additionally, SARS-CoV-2 has undergone numerous mutations throughout the pandemic and different strains have been predominant at various points in time. It is currently unclear if certain strains carry greater risk for acute or chronic cardiovascular complications, as genotype data was not routinely collected in the studies presented. In all, the novelty of the disease limits data availability, and the rapidly evolving landscape of vaccination and viral mutations impairs the generalizability of currently available data to today’s patients.
Conclusions and Future Directions
In summary, acute COVID-19 infection is a significant driver of cardiovascular disease, resulting in direct myocardial injury and increased prevalence of cardiovascular complications including arrhythmia, heart failure, and ischemic heart disease. The presence of myocardial injury is associated with increased mortality and RV dysfunction, as well as increased likelihood of developing post-COVID syndrome. Long-term follow-up has demonstrated higher rates of cerebrovascular disorders, arrhythmia, ischemic heart disease, heart failure, and thrombotic disorders in those who are recovered from acute COVID-19 infection. Additionally, there is emerging evidence of residual RV dysfunction with impaired RV longitudinal strain and myocardial fibrosis on CMR in this population. The presence of fibrosis on CMR and risk for arrhythmia and sudden cardiac death this poses in the long term remains to be seen.
Based on the presented findings and knowledge from prior SARS-CoV pathogens, future areas of study should include ongoing evaluation of the development of cardiovascular disease including diabetes, hypertension, MACE, and heart failure in post-COVID patients compared to the general population. Further evaluation of right ventricular function and presence of myocardial fibrosis in the post-COVID setting would be beneficial to gain better insight into the prevalence of this. Additionally, following patients with RV dysfunction, late gadolinium enhancement, and myocardial fibrosis on imaging studies both during and following acute COVID-19 to elucidate the long-term clinical relevance of these findings will be crucial.
Finally, this review of the available literature regarding long-term outcomes following COVID-19 infection suggests the potential for a substantial increase in global cardiovascular disease burden as SARS-CoV-2 continues to spread throughout the population. Efforts to further characterize the impact of future variants of the SARS-CoV-2 virus on long-term cardiovascular complications, as well as the effect of broad vaccination efforts in reducing them, will be critical in guiding both primary care and cardiovascular clinician’s approaches towards screening and treatment for COVID-associated cardiovascular disease in a world with endemic SARS-CoV-2. With currently available data, the cardiovascular clinician should have a relatively lower threshold to evaluate patients who have recently recovered from acute COVID-19 infection for ventricular dysfunction, arrhythmia, and ischemic heart disease due to a higher incidence of these complications in this population.
Author Contribution
Dr. Tobler, Dr. Pruzansky, and Dr. Slade participated in the review of literature and authoring of the manuscript. Dr. Naderi and Dr. Ambrosy offered additional review of literature and revisions for the manuscript.
Declarations
Conflict of Interest
Dr. Ambrosy is supported by a Mentored Patient-Oriented Research Career Development Award (K23HL150159) through the National Heart, Lung, and Blood Institute, has received relevant research support through grants to his institution from Amarin Pharma, Inc., Abbott, Novartis, Esperion, and Edwards Lifesciences, and modest reimbursement for travel from Novartis. All other authors have no relevant conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Tropical Collection on Coronary Heart Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Diana L. Tobler, Email: diana.l.tobler@kp.org
Alix J. Pruzansky, Email: Alix.J1.Pruzansky@kp.org
Sahar Naderi, Email: sahar.naderi@kp.org.
Andrew P. Ambrosy, Email: andrew.p.ambrosy@kp.org
Justin J. Slade, Email: justin.j.slade@kp.org
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Corman V, Lienau J, Witzenrath M. Coronaviren als Ursache respiratorischer Infektionen. Der Internist. 2019;60(11):1136–1145. doi: 10.1007/s00108-019-00671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wit E, van Doremalen N, Falzarano D, Munster V. SARS and MERS: recent insights into emerging coronaviruses. Nature Reviews Microbiology. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehr A, Channappanavar R, Perlman S. Middle East respiratory syndrome: emergence of a pathogenic human coronavirus. Ann Rev Med. 2017;68(1):387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO coronavirus (COVID-19) dashboard with vaccination data. 2022 [cited 9 February 2022]. Available from: https://covid19.who.int/
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yahia A. Liver injury and dysfunction associated with COVID-19: a review article. Clinical Laboratory. 2022;68(01/2022). [DOI] [PubMed]
- 7.•.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ. 2021;:n693. A cohort study from National Health Service hospitals in the UK which revealed higher rates of major adverse cardiac events in patients discharged home following hospitalization for COVID-19 in comparison to a matched control group from the general population. [DOI] [PMC free article] [PubMed]
- 8.Desai A, Lavelle M, Boursiquot B, Wan E. Long-term complications of COVID-19. Am J Physiol Cell Physiol. 2022;322(1):C1–C11. doi: 10.1152/ajpcell.00375.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Lázaro D, Sánchez-Serrano N, Mielgo-Ayuso J, García-Hernández J, González-Bernal J, Seco-Calvo J. Long COVID a new derivative in the chaos of SARS-CoV-2 infection: the emergent pandemic? Jo Clin Med. 2021;10(24):5799. doi: 10.3390/jcm10245799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker R. COVID-19 and its sequelae: a platform for optimal patient care, discovery and training. J Thrombosis Thrombol. 2021;51(3):587–594. doi: 10.1007/s11239-021-02375-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Maestre-Muñiz M, Arias Á, Mata-Vázquez E, Martín-Toledano M, López-Larramona G, Ruiz-Chicote A et al. Long-term outcomes of patients with coronavirus disease 2019 at one year after hospital discharge. Journal of Clinical Medicine. 2021;10(13):2945. A descriptive cohort study from Spain which describes high rates of cardiovascular morbidity and mortality in individuals within 1 year after hospitalization for acute COVID-19. [DOI] [PMC free article] [PubMed]
- 12.Kuck K. Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz. 2020;45(4):325–326. doi: 10.1007/s00059-020-04924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patone M, Mei X, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nature Medicine. 2021. [DOI] [PMC free article] [PubMed]
- 14.Bhatla A, Mayer M, Adusumalli S, Hyman M, Oh E, Tierney A, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argenziano M, Bruce S, Slater C, Tiao J, Baldwin M, Barr R et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;:m1996. [DOI] [PMC free article] [PubMed]
- 16.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;m1091. [DOI] [PMC free article] [PubMed]
- 17.Zhao Y, Zhao L, Yang X, Wang P. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): a systematic review and meta-analysis. Revi Cardiovasc Med. 2021;22(1):159. doi: 10.31083/j.rcm.2021.01.238. [DOI] [PubMed] [Google Scholar]
- 18.Piazza G, Campia U, Hurwitz S, Snyder J, Rizzo S, Pfeferman M, et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Ame College Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modin D, Claggett B, Sindet-Pedersen C, Lassen M, Skaarup K, Jensen J, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. American Journal of Respiratory and Critical Care Medicine. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehmer T, Kompaniyets L, Lavery A, Hsu J, Ko J, Yusuf H, et al. Association between COVID-19 and myocarditis using hospital-based administrative data — United States, March 2020–January 2021. MMWR Morbidity and Mortality Weekly Report. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Failure. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiology. 2020;5(11):1281. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Arocutipa C, Saucedo-Chinchay J, Imazio M. Pericarditis in patients with COVID-19: a systematic review. J Cardiovasc Med. 2021;22(9):693–700. doi: 10.2459/JCM.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goudot G, Chocron R, Augy J, Gendron N, Khider L, Debuc B, et al. Predictive factor for COVID-19 worsening: insights for high-sensitivity troponin and D-dimer and correlation with right ventricular afterload. Front Med. 2020;7. [DOI] [PMC free article] [PubMed]
- 28.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5(7):811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.•.Weber B, Siddiqi H, Zhou G, Vieira J, Kim A, Rutherford H et al. Relationship between myocardial injury during index hospitalization for SARS-CoV-2 infection and longer-term outcomes. J Am Heart Assoc. 2022;11(1). A prospective outcomes study that found the presence of myocardial injury during index hospitalization is associated with development of post-acute sequelae from COVID-19 illness in the months after acute illness. [DOI] [PMC free article] [PubMed]
- 30.Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106(17):1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweck M, Bularga A, Hahn R, Bing R, Lee K, Chapman A, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud-Elsayed H, Moody W, Bradlow W, Khan-Kheil A, Senior J, Hudsmith L, et al. Echocardiographic findings in patients with COVID-19 pneumonia. Canadian J Cardiol. 2020;36(8):1203–1207. doi: 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuzzi V, Castrichini M, Collini V, Roman-Pognuz E, Di Bella S, Luzzati R, et al. Impaired right ventricular longitudinal strain without pulmonary hypertension in patients who have recovered from COVID-19. Circulation. Cardiovasc Imaging. 2021;14(4). [DOI] [PubMed]
- 34.••.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nature Medicine. 2022. A large cohort study from the Veteran’s Affairs Healthcare database revealing an excess burden of cerebrovascular disorders, arrhythmia, ischemic heart disease, heart failure, and thrombotic disorders between 30 days and 12 months after COVID-19 infection in comparison to control cohorts.
- 35.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Puntmann V, Carerj M, Wieters I, Fahim M, Arendt C, Hoffmann J et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiology. 2020;5(11):1265. A cohort study which investigated cardiac magnetic resonance imaging in patients recently recovered from COVID-19, finding cardiac involvement in 78% of patients and residual myocardial inflammation in 60% of patients. [DOI] [PMC free article] [PubMed]
- 37.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC: Cardiovascular Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Scientific Reports. 2017;7(1). [DOI] [PMC free article] [PubMed]
- 39.Corrales-Medina V, Alvarez K, Weissfeld L, Angus D, Chirinos J, Chang C, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madjid M, Naghavi M, Litovsky S, Casscells S. Influenza and cardiovascular disease. Circulation. 2003;108(22):2730–2736. doi: 10.1161/01.CIR.0000102380.47012.92. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P, Cannon C, Morrow D, Rifai N, Rose L, McCabe C, et al. C-reactive protein levels and outcomes after statin therapy. New England J Med. 2005;352(1):20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 42.Bohula E, Giugliano R, Cannon C, Zhou J, Murphy S, White J, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132(13):1224–1233. doi: 10.1161/CIRCULATIONAHA.115.018381. [DOI] [PubMed] [Google Scholar]
- 43.Alfaddagh A, Martin S, Leucker T, Michos E, Blaha M, Lowenstein C, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prevent Cardiol. 2020;4:100130. doi: 10.1016/j.ajpc.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theofilis P, Sagris M, Antonopoulos A, Oikonomou E, Tsioufis C, Tousoulis D. Inflammatory mediators of platelet activation: focus on atherosclerosis and COVID-19. Intl J Mol Sci. 2021;22(20):11170. doi: 10.3390/ijms222011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavares L, Teixeira M, Garcia C. The inflammatory response triggered by influenza virus: a two edged sword. Inflamm Res. 2016;66(4):283–302. doi: 10.1007/s00011-016-0996-0. [DOI] [PubMed] [Google Scholar]
- 46.Tan L, Komarasamy T, Balasubramaniam RMT, V. Hyperinflammatory immune response and COVID-19: a double edged sword. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed]
- 47.CBC underlying cause of death, 1999–2020 request. 2022 [cited 9 February 2022]. Available from: https://wonder.cdc.gov/ucd-icd10.html
- 48.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bearse M, Hung Y, Krauson A, Bonanno L, Boyraz B, Harris C, et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Modern Pathology. 2021;34(7):1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Intl J Infect Diseas. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra P, Parveen R, Bajpai R, Samim M, Agarwal N. Impact of cardiovascular diseases on severity of COVID-19 patients: a systematic review. Ann Acad Med Singapore. 2021;50(1):52–60. doi: 10.47102/annals-acadmedsg.2020367. [DOI] [PubMed] [Google Scholar]
- 52.Chen D, Li X, Song Q, Hu C, Su F, Dai J, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Network Open. 2020;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RECOVERY Collaborative Group. Horby P, Lim W, et al. Dexamethasone in hospitalized patients with COVID-19. New England J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alessi J, de Oliveira G, Schaan B, Telo G. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr. 2020;12(1). [DOI] [PMC free article] [PubMed]
- 55.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC: Cardiovascular Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Khoury D, Yue Y, Torre-Amione G, Nagueh S. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Euro Heart J. 2007;29(10):1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 57.Bshiebish H, Al-Musawi A, Khudeir S. Role of global longitudinal strain in assessment of left ventricular systolic function in patients with heart failure with preserved ejection fraction. J Saudi Heart Assoc. 2019;31(2):100–105. doi: 10.1016/j.jsha.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowin E, Maron B, Wells S, Burrows A, Firely C, Koethe B, et al. Usefulness of global longitudinal strain to predict heart failure progression in patients with nonobstructive hypertrophic cardiomyopathy. Am J Cardiol. 2021;151:86–92. doi: 10.1016/j.amjcard.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Pellikka P, Naqvi T. The right ventricle. J Am College Cardiol. 2020;76(17):1978–1981. doi: 10.1016/j.jacc.2020.09.529. [DOI] [PMC free article] [PubMed] [Google Scholar]