Abstract
The homogeneous catalytic reduction of a polyoxometalate (POM) by hydrogen gas in aqueous media was investigated for the first time by using a [NiRu] hydrogenase model complex (I) under very mild conditions. By bubbling hydrogen gas into the buffer solution containing I and the Dawson-type POM (IIox), the color of the solution turned from pale yellow to dark blue, suggesting the reduction of IIox. The catalytic and kinetic studies revealed that I acted as an efficient catalyst to yield one-electron-reduced Dawson-type POM (IIred) with a low energy barrier for activating dihydrogen and reducing IIoxvia a hydride complex of I. The process for the one-electron reduction of IIox was confirmed by UV-vis spectroscopy, controlled potential electrolysis, and X-ray photoelectron spectroscopy. POM IIred could stably store protons and electrons and release them by addition of oxidants, demonstrating that POMs acted as redox active mediators for transporting protons and electrons from hydrogen gas to acceptors. The recycle study showed that IIox and IIred could be reduced and oxidized by hydrogen and oxygen gases, respectively, at least five times with >99% yield of reduced species, showing a durable system for extracting protons and electrons from hydrogen gas.
Homogeneous catalytic reduction of polyoxometalates by hydrogen gas with a hydrogenase model complex was investigated for the first time.
Introduction
Polyoxometalates (POMs) are a class of anionic molecular metal oxide clusters that exhibit various unique chemical and physical properties.1 The redox properties of addenda atoms in POMs (W6+, Mo6+, V5+, etc.) have attracted considerable research attention because their highly stable redox states, which are based on the robust POM framework and the ability to delocalize electrons and protons on the anion, enable the exploitation of energy storage materials and redox catalysts, including electrocatalysts and photocatalysts.2 For example, Cronin et al. has recently reported that a Dawson-type POM can be electrochemically reduced up to 18-electrons per molecule, which can be utilized as a high-performance redox flow battery electrolyte and a mediator in an electrolytic cell for on-demand dihydrogen generation.2a Although the reduction of POMs is a key step to store energy or to activate/regenerate catalysts, the reduction of POMs by hydrogen gas has been hardly investigated mainly because the reduction of POMs has been performed by using a (super)stoichiometric amount of organic/inorganic reducing agents in a homogeneous system.3 Therefore, developing a homogeneous catalytic system for reducing POMs by hydrogen gas will provide new chemistry, such as development of hydrogen storage materials, green redox catalysts, fuel cells, and mechanistic studies of hydrogen activation.
Hydrogenases catalyze the reversible oxidation and production of hydrogen gas, wherein the electrons transferring from/to their active sites via iron–sulfur clusters is crucial for their metabolism.4 To imitate their highly-efficient catalytic activities under mild conditions, various types of hydrogenase model complexes have been synthesized to date,5 and we recently reported that the [NiRu] complex could catalytically convert hydrogen gas into protons and electrons through a heterolytic cleavage mechanism.6 Since hydrogen gas is one of the most ecofriendly reducing agents in terms of cost and atom efficiency, developing a catalytic system to extract protons and electrons from hydrogen gas by mimicking hydrogenases is of growing importance.7 However, utilizing extracted protons and electrons as designed is still difficult partly owing to the low catalytic efficiency and the absence of appropriate redox active mediators like iron-sulfur clusters in organisms.8
Herein, we focused on utilizing hydrogenase model complex for reducing POMs by hydrogen gas and for the first time reported the reduction of the α-Dawson-type POM, K6[P2W186+O62] (K6[IIox]), by hydrogen gas with the hydrogenase model complex, [Ni2+(L)Ru2+(H2O){η6-C6(CH3)6}](NO3)2 ([I](NO3)2, L = N,N′-dimethyl-3,7-diazanonane-1,9-dithiolato),6 as a homogeneous catalyst in aqueous media under very mild conditions (pressure of hydrogen gas, ≤0.1 MPa; reaction temperature, 293–333 K). The kinetic study of this catalytic system revealed the small activation energy for activating hydrogen gas and reducing POMs (Ea = 51.2 kJ mol−1). The turnover number (TON) reached to 1975 for 6 h, showing a high-performance homogeneous catalytic system for extracting and storing protons and electrons from hydrogen gas.
Results and discussion
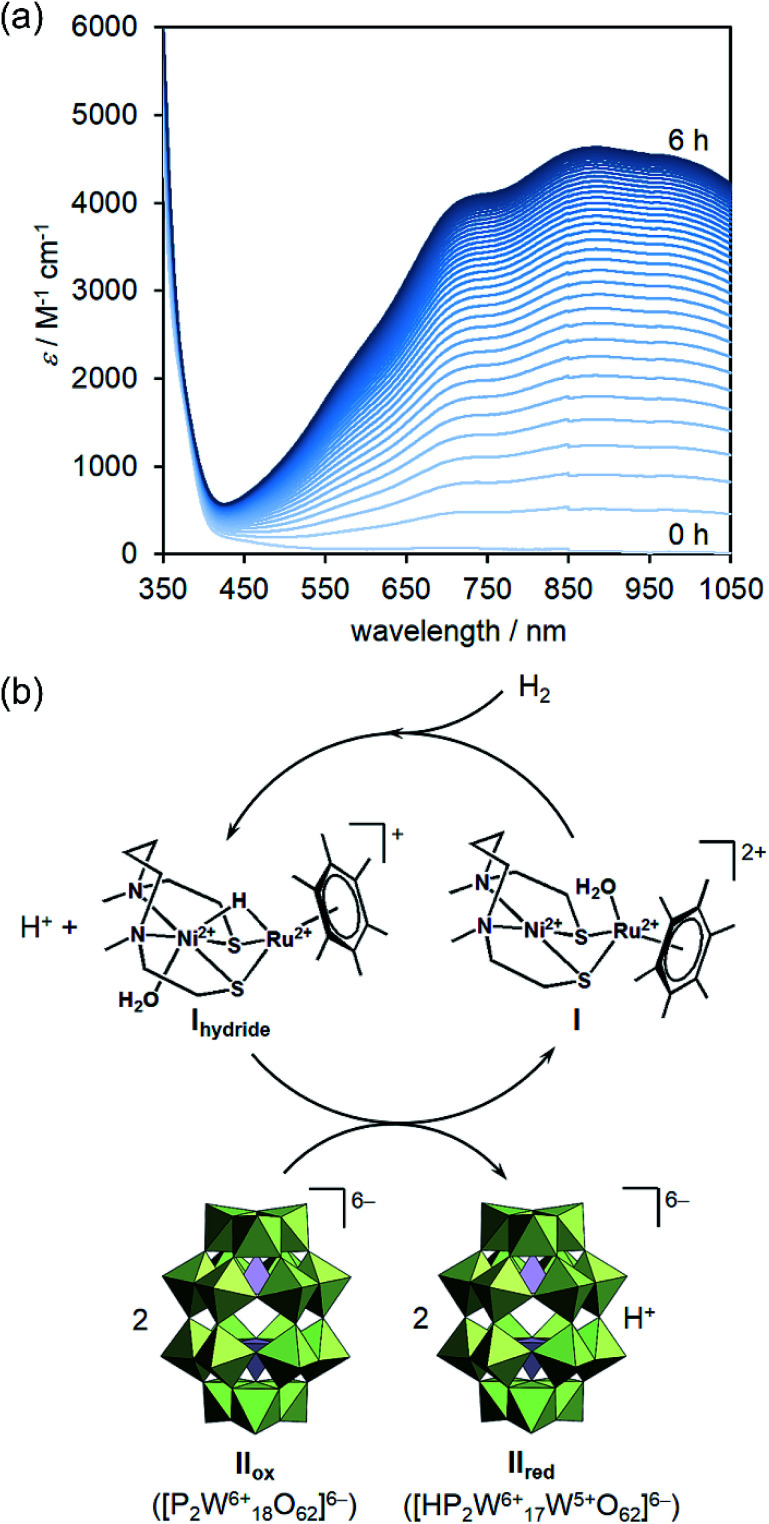
To begin with, hydrogen gas was bubbled into the sodium acetate buffer solution (25 mM, pH 4.1) containing I (0.05 mM) and IIox (0.5 mM) to investigate the catalysis of I at 298 K under Ar. After bubbling hydrogen gas for 1 min, the color of the solution in a sealed quartz cell gradually turned from pale yellow into dark blue. The UV-vis spectra of the solution showed the increase of absorption bands at around 555, 750, 878, and 995 nm assignable to the W5+-to-W6+ intervalence charge transfer (IVCT) process (ε = 4630 M−1 cm−1 at 878 nm, 6 h incubation after bubbling hydrogen gas for 1 min), thereby suggesting the reduction of IIox (Fig. 1a). In contrast, the UV-vis spectra in the range of 550–1050 nm hardly changed in the absence of hydrogen gas, I, or IIox (Fig. S1, ESI†), indicating the I-mediated reduction of IIox by hydrogen gas. Since the UV-vis spectrum measured after 6 h incubation could be superimposed on that of electrochemical one-electron-reduced solution of IIox (Fig. S2, ESI†), the reduced IIox was proved to be a one-electron-reduced species (K6[HP2W176+W5+O62], K6[IIred]). The yield of IIred was calculated using the absorption coefficient at 878 nm and reached to >99% when using catalytic amount of I (10 mol%) (Fig. S3, ESI†).9 The X-ray photoelectron spectroscopy (XPS) spectrum of the vacuum-dried sample of the reaction solution after forming IIred in the W4f region was measured. The spectrum showed three major peaks for W4f7/2 (35.6 eV), W4f5/2 (37.7 eV), and W5p3/2 (41.3 eV) assignable to W6+ species together with three minor peaks for W4f7/2 (34.3 eV), W4f5/2 (36.4 eV), and W5p3/2 (40.1 eV) assignable to W5+ species with 7% area ratio, supporting the one-electron reduction of IIox (Fig. 2). Therefore, based on the above-mentioned results, I could act as homogeneous catalyst to activate hydrogen gas and to give IIred in high yield. It is noteworthy that this system is the first example of homogeneous catalytic reduction of POMs by hydrogen gas.
Fig. 1. (a) UV-vis spectra of the reaction solution measured every 10 min. Reaction conditions: IIox (0.5 mM), I (0.05 mM), sodium acetate buffer (pH 4, 25 mM, 3 mL), 298 K, under Ar (0.1 MPa), the reaction was initiated by bubbling hydrogen gas for 1 min. (b) Proposed catalytic mechanism for reduction of IIox with I by hydrogen gas. The atoms of POMs are represented by polyhedra; [WO6]6− and [WO6]7−: light green, [PO4]3−: gray.
Fig. 2. XPS spectrum of the vacuum-dried sample of the reaction solution after forming IIred. The black dots represent the obtained spectrum. The green and blue lines represent the best fitting curves for W6+ and W5+ species, respectively, and the red line represents the sum of them.
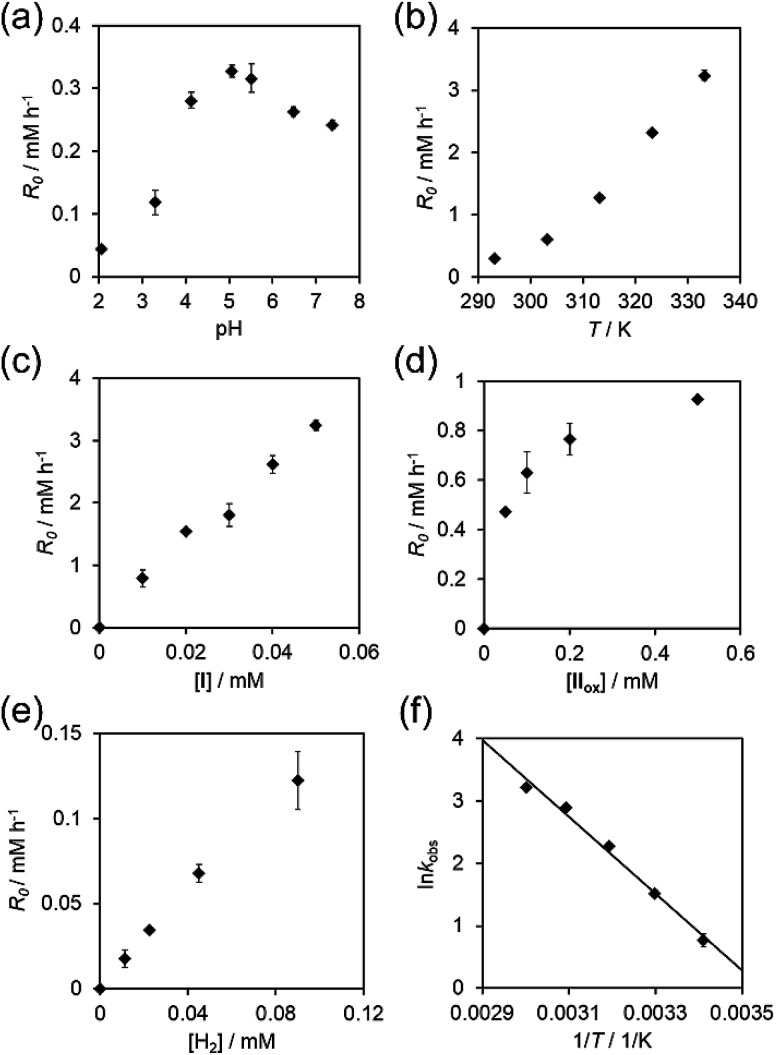
The initial reaction rate R0 (mM h−1), which was calculated by time-course UV-vis spectra at 878 nm, was dependent on pH values and reaction temperatures of buffer solutions (Fig. 3a and b). The plot of pH dependence showed that R0 increased with increasing pH values and reached to the maximum value of 3.3 × 10−1 mM h−1 at pH 5.1, and then, R0 decreased with increasing pH values above 5.1. This type of pH dependence with a maximum was also observed in the studies on the H+/D+ exchange reaction and the reduction of Cu2+ by hydrogen gas with I.10 The plot of temperature dependence showed that R0 increased with increasing reaction temperatures.
Fig. 3. Dependences of the initial reaction rates on (a) pH of the solution, (b) the reaction temperature, (c) the concentration of I, (d) the concentration of IIox, and (e) the concentration of hydrogen gas. (f) Arrhenius plots for I-catalyzed reduction of IIox. The observed rate constants (kobs) were determined from the initial part of the reaction. Line fit: ln kobs = 21.84−6159.3/T.
Next, the catalytic mechanism was investigated using pH 5.1 buffer solution at 333 K. To determine the active species for the reduction of IIox, a hydride complex of I ([Ni2+(H2O)(L)Ru2+(H){η6-C6(CH3)6}](NO3), [Ihydride](NO3)), which was known to be formed by reacting I with hydrogen gas in an acidic solution,6 was added to a deaerated solution of IIox. When adding Ihydride (0.35 μmol) into the solution of IIox (0.5 mM, 3 mL), the color of the solution immediately changed into deep blue. Since the UV-vis spectrum of the resulting solution showed that the yield of IIred reached to 0.63 μmol after 0.5 h incubation, 1.8 equivalents of IIox with respect to Ihydride were reduced to IIred (Fig. S4, ESI†).11 By addition of 1 equivalent of Ihydride with respect to IIox, the UV-vis spectrum exhibited the formation of 1 equivalent of IIred, thus indicating that two-electron reductions (Ihydride + IIox + H+ → I + [H2P2W166+W25+O62]6−) did not occur. This result also supported the formation of one-electron-reduced species in the catalytic study (Fig. S2, ESI†). On the basis of these results and kinetics below, the reaction mechanism for I-catalyzed reduction of IIox by hydrogen gas was proposed as follows (Fig. 1b): Firstly, hydrogen gas was activated by I to form Ihydride and a proton (eqn (1)). Then, 2 equivalents of IIox were reduced by Ihydride using one proton, followed by the regeneration of I and the formation of IIred (eqn (2)).
| I + H2 → Ihydride + H+ | 1 |
| Ihydride + 2IIox + H+ → I + 2IIred | 2 |
The kinetic study on the reduction of IIox was investigated by the time-course UV-vis spectra of the reaction solutions. The first-order dependence of the initial reaction rates R0 on the concentrations of I (0–0.05 mM, Fig. 3c) and hydrogen gas (0–0.09 mM, Fig. 3e) were observed, whereas the saturation kinetics for the dependence of R0 on the concentration of IIox (0–0.05 mM, Fig. 3d) was observed. From the mass balance and steady-state approximation on Ihydride, the overall reduction rate is expressed by the following equation:
 |
3 |
where the initial concentration of I ([I]0) is expressed by [I] + [Ihydride]. On the basis of the kinetic data, the values of rate constants were calculated as follows; k1 = 1.6 × 10 s−1 and k2 = 6.0 × 108 M−1 s−1. The dependences of the reaction rates on the concentrations of I, IIox, and hydrogen gas calculated by eqn (3) were approximately reproduced the experimental data (Fig. S5, ESI†). Since the reaction rate for activating hydrogen gas was much slower than that for reducing IIox according to the obtained rate constants (k1[H2] ≪ k2[IIox][H+]), the rate-determining step was supposed to be the reaction of I with hydrogen gas to form Ihydride and a proton (eqn (1)), which was agreed with the result of the rapid reduction of IIox by Ihydride. The good linearity of the Arrhenius plot was observed to afford the following activation parameters: Ea = 51.2 kJ mol−1, ln A = 21.8, ΔH‡298 K = 48.7 kJ mol−1, ΔS‡298 K = −71.6 J mol−1 K−1, and ΔG‡298 K = 70.1 kJ mol−1 (Fig. 3f). The present activation energy was much lower than free energies for the cleavage of dihydrogen in water (homolytic, 442 kJ mol−1; heterolytic, 143 kJ mol−1),12 showing the successful reduction of energy barrier to activate hydrogen gas by using the catalyst I. The negative value of the activation enthalpy ΔS‡298 K suggested that a bimolecular transition state (hydrogen adduct of I before forming Ihydride) was included in the rate-determining step.13
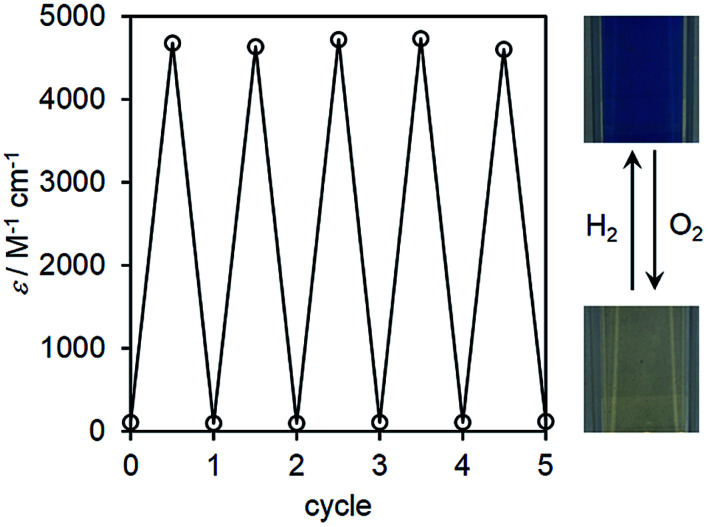
When the reaction was carried out with 0.004 mol% of I at 333 K, the yield of IIred reached to 79% for 6 h, resulted in a high TON of 1975, which was the highest value for the homogeneous catalytic reduction of inorganic substrates by hydrogen gas, to the best of our knowledge (Table S1†). The UV-vis spectrum of the resulting solution hardly changed in a sealed vessel for more than two weeks at room temperature, suggesting that IIox could stably store protons and electrons. By addition of sodium nitrite (15 μmol, 10 equivalents with respect to IIred) into the blue reaction solution containing IIred (0.5 mM, 3 mL), which was formed by I-catalyzed reduction of IIox under hydrogen gas, the color of the solution changed into pale yellow, indicating the reduction of sodium nitrite and oxidation of IIred. The conversion of IIred reached to 99% for 4 h (Fig. S6, ESI†), thus demonstrating the successful re-extraction of protons and electrons viaIIox/IIred as mediators.14 Since IIred could also be oxidized by molecular oxygen, the ability to recycle this system was investigated by bubbling hydrogen gas and oxygen gas alternately. After forming IIred by bubbling hydrogen gas into the reaction solution containing I (0.05 mM) and IIox (0.25 mM), oxygen gas was bubbled to re-oxidize IIred. This process was repeated five times, and the yield of IIred in each step was monitored by measuring the UV-vis spectrum. Although the initial reaction rate gradually decreased with each cycle, IIred was obtained in >99% yield (Fig. 4 and S7, ESI†), indicating that this system was recyclable at least five times with the high stabilities of both the catalyst I and the mediator IIox.
Fig. 4. Reversible changes of the absorption coefficients observed at 878 nm. Insets: images of the reaction solutions under hydrogen and oxygen gases.
Conclusions
In conclusion, a homogeneous catalytic system for extracting and storing protons and electrons from hydrogen gas was developed by using a POM and a hydrogenase model complex for the first time. The present system showed the high yield of reduced POM with ca. 2000 TON, demonstrating a high-performance catalytic system. Extracted protons and electrons could temporary be stored in POMs and released by addition of oxidants, showing that POMs could act as mediators to transport protons and electrons. Moreover, this catalytic system was recyclable at least five times with >99% yield of reduced species. We envisage that these findings would be applied to the development of new catalytic systems and energy storage materials using hydrogen gas under mild conditions.
Experimental section
Materials
Hydrochloric acid (Wako), acetic acid (Wako), sodium acetate (Wako), citric acid monohydrate (Wako), disodium hydrogenphosphate (Wako), sodium dihydrogenphosphate dihydrate (Wako), sodium hydroxide (Wako), sodium nitrite (Wako), chloroform (Wako), and 1,1′-dibenzyl-4,4′-bipyridinium dichloride hydrate (TCI) were purchased and used as received. Compounds [Ni2+(L)Ru2+(H2O){η6-C6(CH3)6}](NO3)2 ([I](NO3)2, L = N,N′-dimethyl-3,7-diazanonane-1,9-dithiolato), [Ni2+(H2O)(L)Ru2+(H){η6-C6(CH3)6}](NO3) ([Ihydride](NO3)), and K6[P2W186+O62] (K6[IIox]) were synthesized according to the reported procedures.6,15 The buffer solutions were prepared using hydrochloric acid (pH 2.06), citric acid/sodium citrate (pH 3.30), acetic acid/sodium acetate (pH 4.13, 5.07, 5.52), or phosphorus acid/sodium phosphate (pH 6.48, 7.38).
Instruments
UV-vis spectra were measured on JASCO V-670. IR spectra were measured on PerkinElmer Spectrum Two. The pH values of the buffer solutions were determined using TOA DK MH-30R pH meter.
Controlled potential electrolysis
The controlled potential electrolysis of IIox (2 mM) in acetate buffer (ca. 60 mL, pH 4, 25 mM) was carried out using an electrolyzer separated by glass frit. Pt electrodes were used as cathode and anode, which were connected to a BAS electrochemical analyzer 600D. Nitrogen gas was bubbled into the solution during the electrolysis with stirring. The solutions of one- and two-electron reduced IIox were prepared by the electrolysis at −0.1 and −0.27 V vs. Ag/AgCl, respectively.
XPS analysis
The XPS analysis was performed using a ULVAC-PHI PHI 5000 VersaProbe II under Al Kα radiation (hν = 1486.6 eV, 15 kV, 25 W). The peak positions were calibrated by the W4f7/2 (35.60 eV) of W6+ atoms in POMs, and the baseline was subtracted by the Shirley method. The curve fitting was performed with the spin–orbit separation ΔEP(W4f5/2–W4f7/2) of 2.1 eV and the intensity ratio I(W4f5/2)/I(W4f7/2) of 0.75.16 The ratio of Lorentzian to Gaussian varied in the range of 50 ± 5%. The sample was prepared as follows: hydrogen gas was bubbled into the aqueous solution (20 mL) containing I (0.05 mM) and IIox (0.25 mM) for 10 min. The UV-vis spectrum of the resulting solution was measured after ca. 1 h incubation at 323 K, showing that the yield of IIred reached to >99%. The resulting solution was dried in vacuo to give a dark blue powder, which was used for the measurement.
Procedures for catalytic reduction of IIox
The buffer solution of I was added to the buffer solution of IIox to give a pale-yellow reaction solution, followed by bubbling Ar for 10 min. The reaction was initiated by bubbling H2 or adding H2-containing aqueous solution into the reaction solution in a sealed quartz cell. In a separate experiment, the concentration of H2 in water was determined by measuring the intensity of absorption band at 600 nm for one-electron reduced 1,1′-dibenzyl-4,4′-bipyridinium dichloride (ε = 7.4 × 103 M−1 cm−1) which was formed by the reaction of 1,1′-dibenzyl-4,4′-bipyridinium dichloride with H2 using Pt as a catalyst. The catalyst I after using catalytic reaction was obtained by the following procedure: Ar was bubbled for 1 h into the reaction solution (100 mL, pH 5, 25 mM) containing I (0.5 mM) and IIox (5 mM), followed by bubbling hydrogen gas for 10 min. The UV-vis spectrum of the resulting solution after 16 h incubation showed that IIox was completely reduced to IIred. Chloroform (100 mL) was added to the resulting solution, and then, the mixture was shaken vigorously to give orange precipitates, which was collected by filtration and measured by the IR spectroscopy. The recycle experiment in a homogeneous system was performed at 333 K by the following procedure: Ar was bubbled for 5 min into the reaction solution (3 mL, pH 5, 25 mM) containing I (0.05 mM) and IIox (0.25 mM), followed by bubbling hydrogen gas for 3 min. The UV-vis spectrum of the resulting solution was measured to determine the yield of IIred after 15–90 min incubation. Then, oxygen gas was bubbled into the resulting solution for 3 min to give a colorless solution after 15–45 min incubation. The UV-vis spectrum of the resulting solution was measured to confirm that IIred was completely re-oxidized to IIox. These processes were repeated five times against the same reaction solution.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported in part by JST CREST Grant Number JPMJCR18R2, Japan, JSPS KAKENHI Grant Numbers 18J00191 and JP26000008 (Specially Promoted Research), and the World Premier International Research Centre Initiative (WPI), Japan. T. M. was supported by the JSPS through a Research Fellowship for Young Scientists.
Electronic supplementary information (ESI) available: Kinetic derivation, Table S1, and Fig. S1–S13. See DOI: 10.1039/c9ra04396a
Notes and references
- (a) Advances in Inorganic Chemistry, ed. R. Eldik and L. Cronin, Elsevier Academic Press, Amsterdam, 2017, vol. 69 [Google Scholar]; (b) Ritchie C. and Boskovic C., Polyoxometalates as Ligands for Functional Lanthanoid Complexes, in Polyoxometalate Chemistry: Some Recent Trends, ed. F. Sécheresse, World Scientific, Singapore, 2013, p. 201 [Google Scholar]; (c) Oms O. Dolbecq A. Mialane P. Chem. Soc. Rev. 2012;41:7497. doi: 10.1039/C2CS35148J. [DOI] [PubMed] [Google Scholar]; (d) Zheng S.-T. Yang G.-Y. Chem. Soc. Rev. 2012;41:7623. doi: 10.1039/C2CS35133A. [DOI] [PubMed] [Google Scholar]; (e) Miras H. N. Yan J. Long D.-L. Cronin L. Chem. Soc. Rev. 2012;41:7403. doi: 10.1039/C2CS35190K. [DOI] [PubMed] [Google Scholar]; (f) Kortz U. Müller A. van Slageren J. Schnack J. Dalal N. S. Dressel M. Coord. Chem. Rev. 2009;253:2315. doi: 10.1016/j.ccr.2009.01.014. [DOI] [Google Scholar]
- (a) Chen J.-J. Symes M. D. Cronin L. Nat. Chem. 2018;10:1042. doi: 10.1038/s41557-018-0109-5. [DOI] [PubMed] [Google Scholar]; (b) Suzuki K. Mizuno N. Yamaguchi K. ACS Catal. 2018;8:10809. doi: 10.1021/acscatal.8b03498. [DOI] [Google Scholar]; (c) Weinstock I. A. Schreiber R. E. Neumann R. Chem. Rev. 2018;118:2680. doi: 10.1021/acs.chemrev.7b00444. [DOI] [PubMed] [Google Scholar]; (d) Rausch B. Symes M. D. Chisholm G. Cronin L. Science. 2014;345:1326. doi: 10.1126/science.1257443. [DOI] [PubMed] [Google Scholar]; (e) Sadakane M. Steckhan E. Chem. Rev. 1998;98:219. doi: 10.1021/cr960403a. [DOI] [PubMed] [Google Scholar]; (f) Sattari D. Hill C. L. J. Am. Chem. Soc. 1993;115:4649. doi: 10.1021/ja00064a028. [DOI] [Google Scholar]
- To the best of our knowledge, the reduction of POMs by hydrogen gas using homogeneous catalysts has never been reported, although a few studies on gas–solid or liquid–solid phase heterogeneous systems have been reported. See: ; Itagaki S. Yamaguchi K. Mizuno N. Chem. Mater. 2011;23:4102. doi: 10.1021/cm2016627. [DOI] [Google Scholar]; Kogan V. Aizenshtat Z. Neumann R. Angew. Chem., Int. Ed. 1999;38:3331. doi: 10.1021/cm2016627. [DOI] [PubMed] [Google Scholar]; Mizuno N. Katamura K. Yoneda Y. Misono M. J. Catal. 1983;83:384. doi: 10.1021/cm2016627. [DOI] [Google Scholar]; Katamura K. Nakamura T. Sakata K. Misono M. Yoneda Y. Chem. Lett. 1981;10:89. doi: 10.1021/cm2016627. [DOI] [Google Scholar]
- (a) Lubitz W. Ogata H. Rüdiger O. Reijerse E. Chem. Rev. 2014;114:4081. doi: 10.1021/cr4005814. [DOI] [PubMed] [Google Scholar]; (b) Fontecilla-Camps J. C. Volbeda A. Cavazza C. Nicolet Y. Chem. Rev. 2007;107:4273. doi: 10.1021/cr050195z. [DOI] [PubMed] [Google Scholar]; (c) Evans D. J. Pickett C. J. Chem. Soc. Rev. 2003;32:268. doi: 10.1039/B201317G. [DOI] [PubMed] [Google Scholar]
- (a) Ogo S. Coord. Chem. Rev. 2017;334:43. doi: 10.1016/j.ccr.2016.07.001. [DOI] [Google Scholar]; (b) Ohki Y. Tatsumi K. Eur. J. Inorg. Chem. 2011:973. doi: 10.1002/ejic.201001087. [DOI] [Google Scholar]; (c) Tard C. Pickett C. J. Chem. Rev. 2009;109:2245. doi: 10.1021/cr800542q. [DOI] [PubMed] [Google Scholar]; (d) Gloaguen F. Rauchfuss T. B. Chem. Soc. Rev. 2009;38:100. doi: 10.1039/B801796B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogo S. Kabe R. Uehara K. Kure B. Nishimura T. Menon S. C. Harada R. Fukuzumi S. Higuchi Y. Ohhara T. Tamada T. Kuroki R. Science. 2007;316:585. doi: 10.1126/science.1138751. [DOI] [PubMed] [Google Scholar]
- (a) Ogo S. Chem. Commun. 2009:3317. doi: 10.1039/B900297A. [DOI] [PubMed] [Google Scholar]; (b) Vincent K. A. Parkin A. Armstrong F. A. Chem. Rev. 2007;107:4366. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]
- A redox active organometallic mediator as a co-catalyst for nitrogen fixation has been reported recently. ; Chalkley M. J. Del Castillo T. J. Matson B. D. Peters J. C. J. Am. Chem. Soc. 2018;140:6122. doi: 10.1021/jacs.8b02335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Although it was hard to isolate catalyst I after the reaction, the IR spectrum of the ionic pair which was obtained by addition of chloroform into the reaction solution suggested that the structure of I was maintained during the catalytic reaction (Fig. S8, ESI†).
- (a) Matsumoto T. Kure B. Ogo S. Chem. Lett. 2008;37:970. doi: 10.1246/cl.2008.970. [DOI] [Google Scholar]; (b) Kure B. Matsumoto T. Ichikawa K. Fukuzumi S. Higuchi Y. Yagi T. Ogo S. Dalton Trans. 2008:4747. doi: 10.1039/B807555G. [DOI] [PubMed] [Google Scholar]
- Orange precipitates were formed after addition of Ihydride. The IR spectrum of the precipitate showed absorption bands assignable to I and IIox, indicating that a regenerated cationic [I(NO3)]+ or [I]2+ was reacted with an anionic [IIox]6− to form insoluble materials under a superstoichiometric condition. When bubbling hydrogen gas into the resulting solution, orange precipitates were dissolved immediately, followed by the reduction of remained IIox using regenerated I (Fig. S4, ESI†), indicating that the formation of ionic pairs of catalysts and POMs did not affect the catalytic cycle in the presence of hydrogen gas.
- Connelly S. J. Wiedner E. S. Appel A. M. Dalton Trans. 2015;44:5933. doi: 10.1039/C4DT03841J. [DOI] [PubMed] [Google Scholar]
- Kim K. Kishima T. Matsumoto T. Nakai H. Ogo S. Organometallics. 2013;32:79. doi: 10.1021/om300833m. [DOI] [Google Scholar]
- When adding 150 μmol of sodium nitrite, the conversion of IIred reached to >99% within 10 min.
- Contant R. Inorg. Synth. 1990;27:10. [Google Scholar]
- Xie F. Y. Gong L. Liu X. Tao Y. T. Zhang W. H. Chen S. H. Meng H. Chen J. J. Electron Spectrosc. Relat. Phenom. 2012;185:112. doi: 10.1016/j.elspec.2012.01.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.