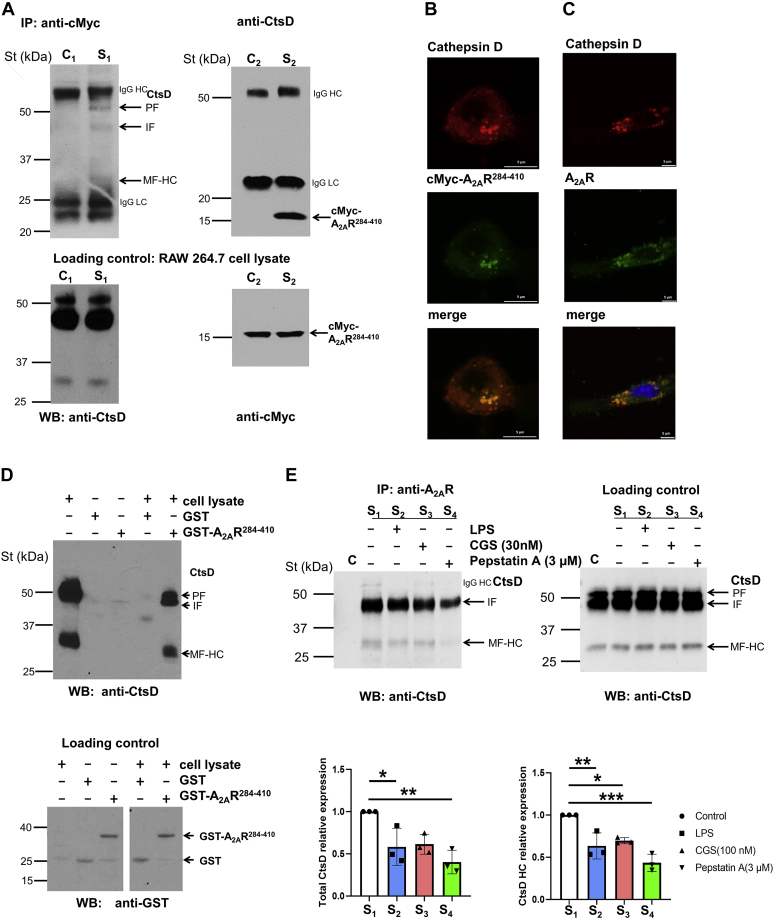
Figure 2.
Validation of the interaction between CtsD and the A2AR in macrophages.A, RAW 264.7 cells were transfected with a pCMV-cMyc-A2AR284–410 construct. The recombinant cMyc-A2AR284–410 and the endogenous CtsD were coimmunoprecipitated in RAW 264.7 cells. Specific bands were detected in the immune complex by WB using cMyc and CtsD antibodies. The IP was carried out using 10 μg of specific antibody in the sample (S1 and S2) and the same amount of isotype control IgG1 (C1) and goat control serum (C2) in the control experiments. For loading controls (lower panel), 10 μg of total protein were analyzed in each lane. CtsD PF, proform; IF, intermediate form; MF-HC, mature form of heavy chain; St denotes Precision Plus Protein Dual Color Standards; and IgG HC, LC denotes immunoglobulin heavy and light chains, respectively. B, colocalization of cMyc-A2AR284–410 and endogenous CtsD in RAW 264.7 cells by IS. CtsD-specific and cMyc-specific primary antibodies were used to identify target proteins. The secondary antibodies were labeled with Alexa-647 (red) and Alexa-546 (green), respectively, and specific labeling was visualized by confocal microscopy (Leica TCS SP8). C, colocalization of endogenous A2AR and CtsD was detected in mouse IPMΦ cells by IS. CtsD-specific and A2AR-specific primary antibodies were used for the identification of target proteins. The secondary antibodies were labeled with Alexa-647 (red) and Alexa-546 (green), respectively, and the nucleus was stained with DAPI. The images were captured by confocal microscopy (Leica TCS SP8). D, endogenous CtsD was pulled down by recombinant GST-A2AR284–410 but not by GST alone in the cell lysate of IPMΦs. In the cell lysate, 10 μg of total protein were incubated with GST and GST-A2AR284–410 recombinant proteins, and the binding of CtsD was detected using CtsD-specific antibody. GST or GST-A2AR284–410 recombinant proteins in each lane were detected with GST-specific antibodies (lower panel). CtsD PF, proform; IF, intermediate form; MF-HC, mature form of heavy chain; St denotes Precision Plus Protein Dual Color Standards. E, the endogenous A2AR and the CtsD were coimmunoprecipitated in RAW 264.7 cells. Specific bands were detected in the immune complex by WB using CtsD antibodies. The IP was carried out using 8.5 μg of A2AR-specific antibody in the sample (S1–S4), and antibody was not added in the control experiment (C). For loading controls (right panel), 10 μg of total protein were analyzed in each lane. CtsD PF, proform; IF, intermediate form; MF-HC, mature form of heavy chain; St denotes Precision Plus Protein Dual Color Standards; IgG HC, immunoglobulin heavy chain, respectively. Densitometry analysis data are presented as mean ± SD of three independent experiments. Values from ANOVA: F = 9.217 and p = 0.0057 for total CtsD and F = 18.59 and p = 0.0006 for CtsD HC. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus control (in the absence of A2AR-specific antibody). A2AR, adenosine A2A receptor; CtsD, cathepsin D; DAPI, 4′,6-diamidino-2-phenylindole; GST, glutathione-S-transferase; IP, immunoprecipitation; IPMΦ, mouse peritoneal macrophage; IS, immunostaining; pCMV, plasmid cytomegalovirus; WB, Western blot.