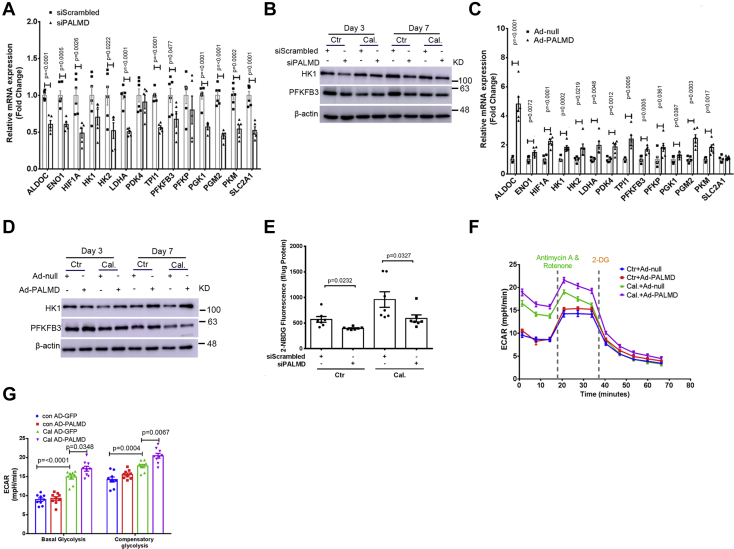
Figure 4.
PALMD enhances glycolysis in hVICs. hVICs were transfected with 20 nM siScrambled or siPALMD and treated with control medium (Ctr) or calcifying medium (Cal.) for 48 h. A, RT-qPCR showing expression of the key glycolytic genes in hVICs with PALMD depletion after 48 h, n = 5. B, representative Western blotting images for HK1, PFKFB3, and β-actin protein expression in hVICs with PALMD depletion at day 3 and day 7, n = 4 or 5. C, RT-qPCR showing expression of the key glycolytic genes in hVICs infected with Ad-null (MOI = 100) or Ad-PALMD (MOI = 100) for 48 h, n = 6. D, representative Western blotting images for HK1, PFKFB3, and β-actin protein expression in hVICs with PALMD overexpression at day 3 and day 7, n = 3. E, normalized fluorescence of the glucose analog 2-NBDG (fl/μg protein) determined after 48 h in hVICs with silencing of PALMD, n = 7. F and G, the glycolysis rate assay using the Seahorse in hVICs with PALMD overexpression after 48 h, n = 9. Data are presented as mean ± SEM, and statistical significance was analyzed by a two-tailed unpaired Student’s t test or one-way ANOVA followed by Tukey's multiple comparisons test. hVIC, human valve interstitial cell.