-
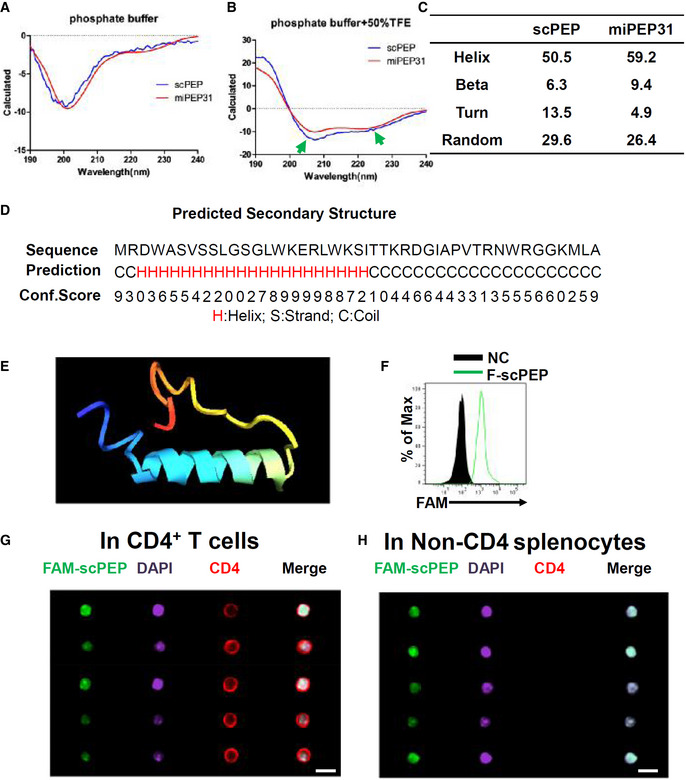
A, B
miPEP31 or scPEP with the concentration of 100 μg/ml in phosphate buffer (A) or phosphate buffer with 50% TFE (B) was analyzed by circular dichroism. The ratio between molar ellipticity at 222 nm and 207 nm was used to confirm an α‐helical structure of peptides. The arrows indicate the α‐helical structure of miPEP31 and scPEP.
-
C
Secondary structures of miPEP31 and scPEP.
-
D
The secondary structure of miPEP31 was predicted by I‐TASSER. H, S, and C stands for helix, stand, or coil tend of different residues.
-
E
The structure model of miPEP31 predicted by I‐TASSER.
-
F
CD4+ T cells were activated with anti‐CD3/28 and treated with 10 μM FAM‐scPEP for 24 h, the fluorescein was detected by flow cytometry.
-
G, H
C57BL/6 mice were injected intravenously with 100 μg FAM‐labeled scPEP. Splenocytes were isolated and stained for CD4 and DAPI. Imaging flow cytometry analysis of FAM‐scPEP in CD4+ T cells (G) and non‐CD4 cells (H). Shown in (G, H) are representative of two independent experiments, scale bar 10 μm.