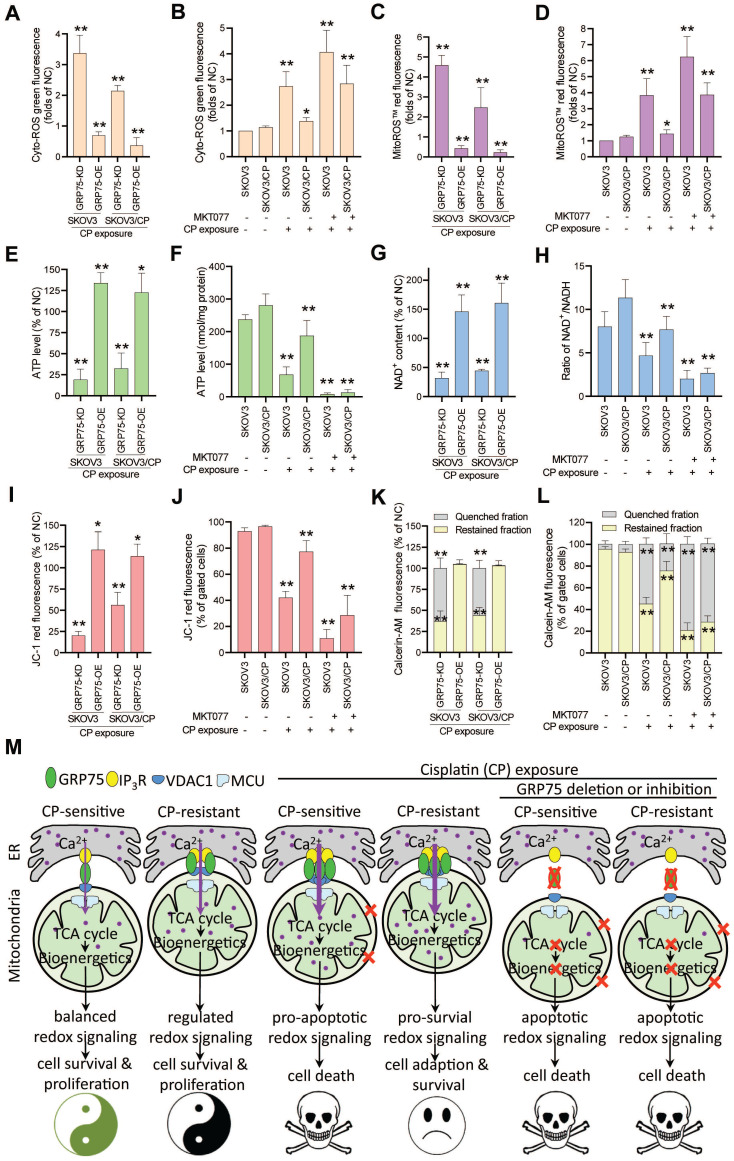
Figure 7.
GRP75-KD or inhibition accelerated CP-induced mitochondrial dysfunction. (A, C, E, G, I, K) GRP75-expression-changed OC cells were treated with CP (4ug/mL) for 48h. (B, D, F, H, J. L) CP-sensitive and -resistant OC cells were treated with CP (4 µg/mL) and/or MKT077 (40 µM) for 48h. (A, B) iROS: level was determined by Amplite™ ROS Green staining and measured by a microplate reader. (C, D) mtROS level was determined by MitoROS™ 580 staining and measured by a microplate reader. (E, F) The level of intracellular ATP was measured by using the enhanced ATP kit. The ATP level in the NC group of OC cells was set as 100%. (G, H) Intracellular NAD+/NADH ratio was determined by using the NAD+/NADH assay kit. The NAD+ level in NC group of OC cells was set as 100%. (I, J) Change in mitochondrial membrane potential was determined by flow cytometry-based measurement of red fluorescence. The ΔΨm in the NC group of OC cells was set as 100%. (K, L) Quantitative analysis of calcein-AM fluorescent intensity in the presence (quenched) and absence (retained) of CoCl2 in OC cells as determined by flow cytometry. The relative calcein fluorescence in the NC group of OC cells was set as 100%. All data represent mean ± SD from three independent experiments. *P < 0.05, **P < 0.01 compared to the NC group or untreated OC cells. (M) Working model: GRP75-faciliated MAM integrity represents a checkpoint in regulating the CP-resistance. GRP75-mediated MAM formation boosts ER-mitochondrial Ca2+ fluxes, and drives mitochondrial bioenergetics and ROS production, which control the balance of pro-survival and pro-apoptotic signals in OC cells. (1) Low-level constitutive ER-to-mitochondria Ca2+ fluxes maintain the TCA cycle running, which sustains energy production (ATP), redox homeostasis (NADH generation), anabolic pathways (biosynthesis of macromolecules), and the survival and proliferation of OC cells. (2) CP-exposure elicits the damage to nDNA and mtDNA, causes Ca2+ overload-release from ER to mitochondria through increased MAM formation, leads to pro-apoptotic mtROS production, mPTP opening and cytochrome C release, triggers activation of Caspases, and induces apoptosis in CP-sensitive OC cells. (3) CP-resistant OC cells distinctively manage the [Ca2+]m uptake to stimulate Ca2+-dependent mitochondrial metabolism and pro-survival mtROS level, while avoiding the Ca2+-triggered cell death by fine-tuning GRP75-mediated MAM formation. This probably involves metabolic reprogramming and up-regulated antioxidant enzymes to prevent deleterious [Ca2+]m and mtROS accumulation. (4) GRP75-deficiency abrogates VDAC1-IP3R1 interaction and ER-mitochondrial coupling, causes ER-to-mitochondria Ca2+ transfer-interrupted, mitochondrial dysfunction, compromised OXPHOS (NADH decline), severe bioenergetic crisis (ATP depletion), and eventually results in apoptotic death in CP-sensitive and -resistant OC cells. In these GRP75-depleted cells, CP exposure-induced catastrophic oxidative stress accelerates the mitochondrial dysfunction and cell death.