Abstract
Introduction:
The radioisotopes of bromine are uniquely suitable radiolabels for small molecule theranostic radiopharmaceuticals but are of limited availability due to production challenges. Significantly improved methods were developed for the production and radiochemical isolation of clinical quality 76Br, 77Br, and 80mBr. The radiochemical quality of the radiobromine produced using these methods was tested through the synthesis of a novel 77Br-labeled inhibitor of poly (ADP-ribose) polymerase-1 (PARP-1), a DNA damage response protein.
Methods:
76Br, 77Br, and 80mBr were produced in high radionuclidic purity via the proton irradiation of novel isotopically-enriched Co76Se, Co77Se, and Co80Se intermetallic targets, respectively. Radiobromine was isolated through thermal chromatographic distillation in a vertical furnace assembly. The 77Br-labeled PARP inhibitor was synthesized via copper-mediated aryl boronic ester radiobromination.
Results:
Cyclotron production yields were 103 ± 10 MBq∙μA−1∙h−1 for 76Br, 88 ± 10 MBq∙μA−1∙h−1 for 80mBr at 16 MeV and 17 ± 1 MBq∙μA−1∙h−1 for 77Br at 13 MeV. Radiobromide isolation yields were 76 ± 11% in a small volume of aqueous solution. The synthesized 77Br-labeled PARP-1 inhibitor had a measured apparent molar activity up to 700 GBq/μmol at end of synthesis.
Conclusions:
A novel selenium alloy target enabled clinical-scale production of 76Br, 77Br, and 80mBr with high apparent molar activities, which was used to for the production of a new 77Br-labeled inhibitor of PARP-1.
Advances in Knowledge:
New methods for the cyclotron production and isolation of radiobromine improved the production capacity of 77Br by a factor of three and 76Br by a factor of six compared with previous methods.
Implications for Patient Care:
Preclinical translational research of 77Br-based Auger electron radiotherapeutics, such as those targeting PARP-1, will require the production of GBq-scale 77Br, which necessitates next-generation, high-yielding, isotopically-enriched cyclotron targets, such as the novel intermetallic Co77Se.
Keywords: bromine-77, bromine-76, bromine-80m, Auger radionuclide therapy, positron emission tomography, PARP-1 inhibitor
INTRODUCTION
The radioisotopes of bromine with medical relevance include the diagnostic positron-emitter 76Br (t1/2 = 16.2 h) and therapeutic Auger-emitters 77Br (t1/2 = 57.0 h) and 80mBr (t1/2 = 4.42 h). Radiobromine is organochemically versatile, participating in labeling reactions including oxidative electrophilic radiobrominations using alkyl tin precursors [1] and nucleophilic aromatic radiobrominations using diaryliodonium salt [2] and aryl boron [3] precursors. Many radiobrominated compounds have been investigated, including thymidine analogues bromodeoxyuridine ([77Br]BrUdR) [4] and fluoro-bromo- arabanofurosyl-uracil ([76Br]FBAU) [5], steroid receptor ligand methoxybromoestradiol ([77Br]MBE) [6], peptides [7] and proteins [8]. Additionally, radiobromine has an advantage over the radioisotopes of iodine in that the C–Br bond is more stable than C–I bond resulting in less dehalogenation of radiolabeled compounds in vivo. Rather than accumulate in the thyroid like iodide, radiobromide ions liberated due to in vivo dehalogenation remain distributed primarily in the blood pool, with an excretion rate of ~10 days in humans [9], resulting in a more diffuse dosimetric burden. These properties make bromine radioisotopes uniquely suited for incorporation into small molecule theranostic agents.
Small biomedical cyclotrons produce the medical radioisotopes of bromine via the 77Se(p,n)77Br, 76Se(p,n)76Br, and 80Se(p,n)80mBr nuclear reactions. However, selenium’s low electrical and thermal conductivity, boiling point, and high vapor pressure significantly limit its tolerance to irradiation, even with modest proton intensities. The cyclotron irradiation of binary intermetallic compounds of transition metals and selenium was pioneered in Groningen [10] using Cu2Se. The use of Cu2Se was later adapted for use with isotopically enriched Cu276Se [11,12] and Cu277Se [12] for the production of radionuclidically pure 76Br and 77Br, respectively. More recently, investigations of the intermetallic compounds NiSe [13,14] and ZnSe [15] are reported, but only with selenium of natural isotopic composition. Despite this progress, 76Br production capacity remains limited to ~2 GBq and 77Br to ~0.7 GBq per three hour irradiation, dramatically less than the amounts needed for clinical studies. This low 76,77Br yield is primarily due to the thermal limitations of the selenium target resulting in a maximum proton irradiation intensity of 15 – 20 μA [11,12], a fraction of modern medical cyclotrons’ >100 μA capabilities. Cu2Se and NiSe cyclotron targets are also problematic because of co-production of large quantities of gamma-emitting 63Zn (t1/2 = 38.1 m) and 60Cu (t1/2 = 23.7 m), respectively. The proton activation of naturally monoisotopic cobalt is dosimetrically advantageous, producing small amounts of low radiation dose-emitting 59Ni (t1/2 = 76,000 y) and 58gCo (t1/2 = 70.9 d). This work aims to mitigate the thermal and dosimetric limitations of radiobromine production targets through the use of a previously unexplored intermetallic, cobalt selenide (CoSe).
Selenium intermetallics release radiobromine when heated, enabling radiobromine recovery via thermal chromatographic distillation and avoiding time consuming target dissolution and recycling of costly enriched materials. So-called “dry distillation” isolates 124I [16,17] and 211At [18–21] from tellurium and bismuth targets, respectively, with horizontal distillation assemblies that cool slowly after distillation. A compact, easily-assembled vertical distillation assembly that cools rapidly, such as that used for isolating 94mTc [22], is reported here for the isolation of 77/76/80mBr.
Radiolabeled inhibitors of the DNA damage response protein, poly ADP ribose polymerase 1 (PARP-1) have been evaluated for non-invasive quantification of PARP-1 expression for patient stratification and treatment response monitoring of PARP inhibitor chemotherapy [23,24]. Additionally, the pharmacological mechanism of action brings PARP inhibitors in close proximity to cancer cell DNA [25], enabling targeted Auger-electron radiotherapy. Recent radiochemistry reports of 77Br-labeled PARP inhibitors [2,3] demonstrate the field is moving in this direction. The radiochemical quality of the radiobromine produced in this work was evaluated by copper-mediated aryl boronic ester bromination, synthesizing a novel 77Br-labeled derivative of the PARP-1 inhibitor, rucaparib.
MATERIALS AND METHODS
Materials
Cobalt powder (Alfa Aesar, 1.6 μm, 99.8%), natural enrichment selenium powder (Acros Organics, 200 mesh, 99.5%), and >99.6% isotopically enriched 76Se, 77Se, and 80Se powders (Isoflex USA) of isotopic abundance summarized in Table S1 were used for the synthesis of intermetallic CoSe. 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one (pre-KX1-Bpin) and 1-(4-iodophenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one (KX-1) were synthesized as previously described [26]. Copper catalyst (tetrakis(pyridine)copper (II) triflate; Cu(py)4(OTf)2) and ligand (3,4,7,8-tetramethyl-1,10-phenanthroline; Lig) were obtained from Sigma Aldrich. Sep-Pak QMA Plus Light (Waters, QMA light) cartridges were prepared with 10 mL of 1 M KHCO3 or 0.5 M Na2SO4 and 10 mL water, and Sep-Pak C18 Plus light (Waters, C18 light) cartridges were prepared with 5 mL ethanol and 10 mL water prior to use. All other chemicals were purchased from Sigma Aldrich and used as received.
Production of CoSe cyclotron targets
Cobalt selenide was formed from equal parts elemental cobalt and selenium by heating to 1200 °C in an evacuated quartz ampule. CoSe cyclotron targets were formed by hot pressing CoSe at ~1100 °C into a pocketed (⌀ = 9.5 mm, 1 mm deep) niobium disc (⌀ = 19 mm, 2 mm thick) using the vertical furnace assembly shown in Figure 1. Detailed descriptions of these metallurgical processes are given in the supplementary material. A prepared CoSe cyclotron target was analyzed by X-ray diffraction using a Bruker D8 Discovery X-ray Diffractometer with a Cu Kα X-ray source (1.54 Å, 2 mm cone diameter) and a Vantec-500 detector at 0.6 sample rotations per minute.
Figure 1.
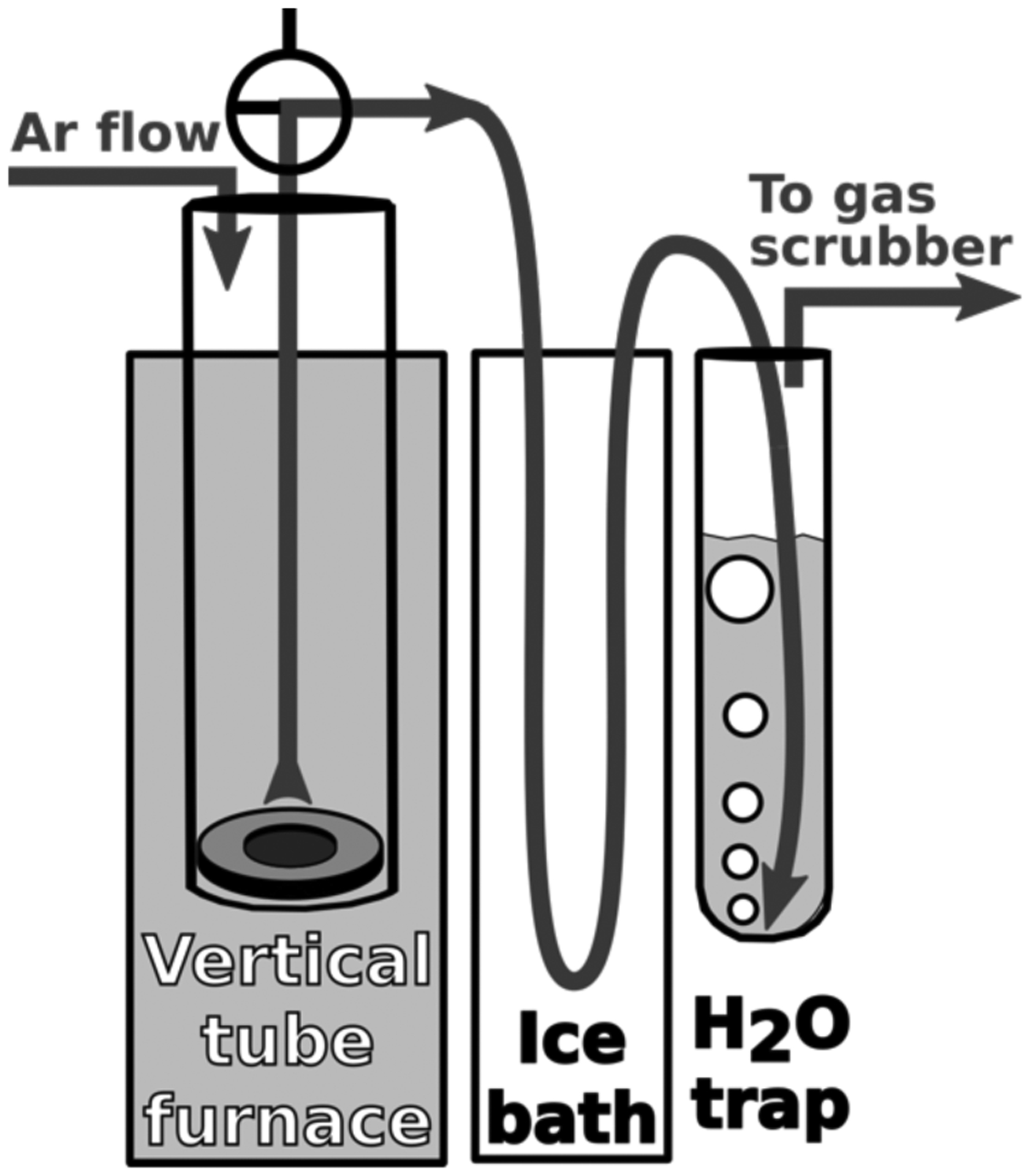
Radiobromine furnace assembly with CoSe heated inside quartz tube (left).
Cyclotron production of radiobromine
CoSe with natSe, 80Se, 76Se, or 77Se constituents on niobium backings was irradiated with 5 – 40 μA of 11 – 16 MeV protons on the University of Wisconsin GE PETtrace cyclotron. A water jet cooled the back of the niobium disc using an ARTMS QIS solid target system (Vancouver, Canada). Radiobromine production yields and radionuclidic purities were measured at four proton energies by employing a water-cooled degrader foil positioned 3.6 cm away from the face of the CoSe target. Molybdenum and tungsten foils (Alfa Aesar) degraded the 16 MeV primary beam to 13, 12, or 11 MeV proton energy with a 0.10 mm W foil, a 0.20 mm Mo foil, or a 0.25 mm Mo foil, respectively, based on calculations performed with SRIM-2013.00 [27]. High purity germanium (HPGe) spectrometry quantified radioactivity in mixed radionuclide sources and dose calibrator measurements (Capintec CRC 15R, setting #690÷2 for 76Br, #121 for 77Br, and #170 for 80mBr) quantified activity in fractions following radiochemical separation.
Radiochemical isolation of radiobromine
Radiobromine thermal chromatographic distillation from irradiated CoSe targets occurred in the same furnace assembly shown in Figure 1, as detailed in the supplementary material. Briefly, the irradiated CoSe was sealed in the assembly and lowered into a tube furnace preheated to 1050 °C. Multiple collimated radiation detectors monitored the progress of the distillation. Following 5 – 15 minutes of heating, the tube was removed from the furnace and quenched in water. After cooling and venting, warm water rinsed the outlet gas flow path into the H2O trap. The water was passed through a prepared QMA light cartridge, trapping the radiobromide, followed by its elution with 700 μL of 20 mM K2SO4 or 0.1 M NH4OH in 1:1::MeCN:H2O. HPGe spectrometry and dose calibrator measurements assessed the radiochemical yield of the distillation process.
Radiosynthesis of 77Br-labeled PARP inhibitor
The copper-mediated aryl boronic ester bromination reaction shown in Figure 2 evaluated the radiochemical quality of the [77Br]bromide by using 1 μmol pre-KX1-Bpin with varying solvent volume and composition, K2SO4 concentration, and temperature. Reactions were purified by diluting in 15 mL water, loading on a prepared C18 light cartridge, rinsing with 10 mL water, and eluting crude product in 700 μL ethanol. Following a 1:1 dilution with water, preparative HPLC purified the product (Kinetix XB-C18, 5 μm, 100 Å, 10×250 mm, 4 mL/min 40:60 ∷ MeCN:0.1 M ammonium formate, pH 4.5). A final C18 light cartridge purification formulated the product in a small volume ethanol solution. Dose calibrator measurements of purified fractions determined the radiochemical conversion. Preparative HPLC injections of 100 – 500 pmol of stable, iodinated KX1 estimated the 77Br-labeled PARP inhibitor (77Br-PARPi) mass versus 254 nm absorbance calibration curve.
Figure 2.
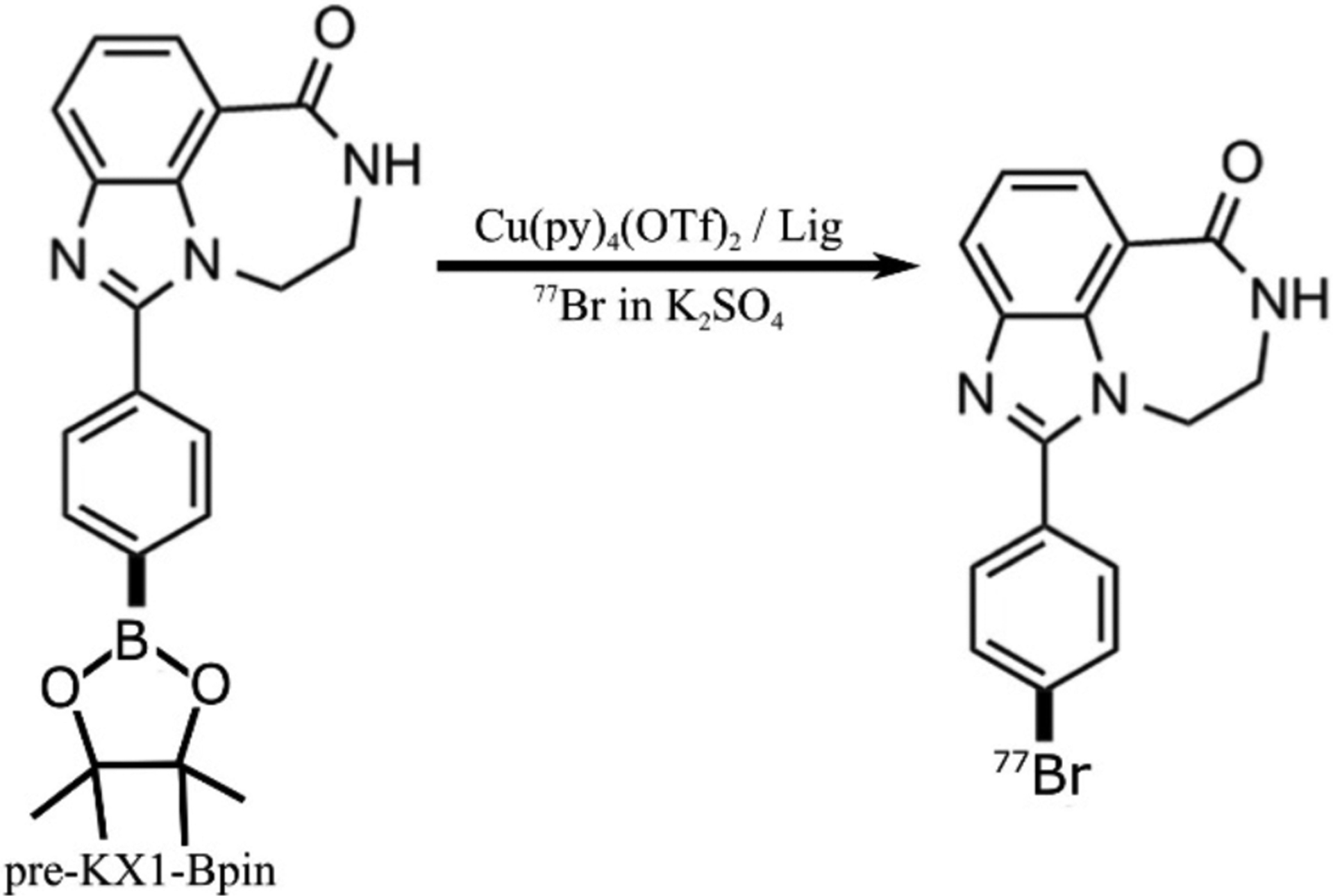
Radiosynthesis of 77Br-labeled PARP-1 inhibitor.
RESULTS
Production of CoSe cyclotron targets
Elemental cobalt and selenium powder readily fused into solid pieces (270 ± 20 mg) in 1 hour at 1200 °C inside a vacuum ampule. Typical mass losses to the ampule walls were 6 ± 4% (n=10). CoSe cyclotron targets contained 180 – 220 mg of CoSe in a 9.5 mm diameter pocket and exhibit the X-ray diffraction pattern shown in Figure S2.
Cyclotron production of radiobromine
Water-cooled CoSe cyclotron targets withstood proton irradiation at all investigated proton energies (11 – 16 MeV) and intensities (5 – 40 μA). The radiobromine production rate was consistent between 10 and 40 μA (Figure S3), indicating that CoSe targets retain radiobromine up to at least 640 W of power deposition (at 40 μA). Radiobromine yields [28] from CoSe targets are shown in Table 1 and Figure 3 with end of bombardment (EoB) radionuclidic purities in Table 2. 58gCo was co-produced at 140 ± 50 kBq∙μA−1∙h−1 at 16 MeV (n=4) and 20 ± 10 kBq∙μA−1∙h−1 at 13 MeV (n=3).
Table 1.
Production yield of 82Br, 80mBr, 76Br, and 77Br from various isotopic compositions of CoSe targets at four proton energies (Ep). Reported uncertainties represent standard deviations of multiple irradiations or are estimated when n=1. Limits of detection calculated from HPGe spectra [29] are reported. In some cases (denoted as n/a), HPGe measurements were too late to quantify short-lived 80mBr.
| Ep (MeV) | Target | n | Physical yield (MBq·μA−1·h−1) | |||
|---|---|---|---|---|---|---|
| 82Br | 80mBr | 76Br | 77Br | |||
| 16 | ConatSe | 4 | 2.0 ± 0.3 | 62 ± 7 | 9.9 ± 0.9 | 2.8 ± 0.4 |
| Co80Se | 12 | 0.0011 ± 0.0001 | 103 ± 10 | 0.0072 ± 0.0005 | 0.006 ± 0.005 | |
| Co77Se | 1 | <0.07 | n/a | 12 ± ~1 | 23 ± ~2 | |
| Co76Se | 2 | <0.06 | n/a | 88 ± 10 | 0.05 ± ~0.005 | |
| Co80Se | 2 | 0.0015 ± 0.0002 | 77 ± 7 | 0.0040 ± 0.0005 | <0.002 | |
| 13 | Co77Se | 3 | <0.02 | <0.3 | 0.07 ± 0.01 | 17 ± 1 |
| Co76Se | 1 | <0.004 | n/a | 50 ± ~5 | 0.1 ± ~0.01 | |
| 12 | Co77Se | 2 | <0.002 | <0.5 | 0.048 ± 0.001 | 13.1 ± 0.5 |
| 11 | Co80Se | 8 | 0.0015 ± 0.0003 | 48 ± 3 | 0.0010 ± 0.0001 | <0.002 |
Figure 3.
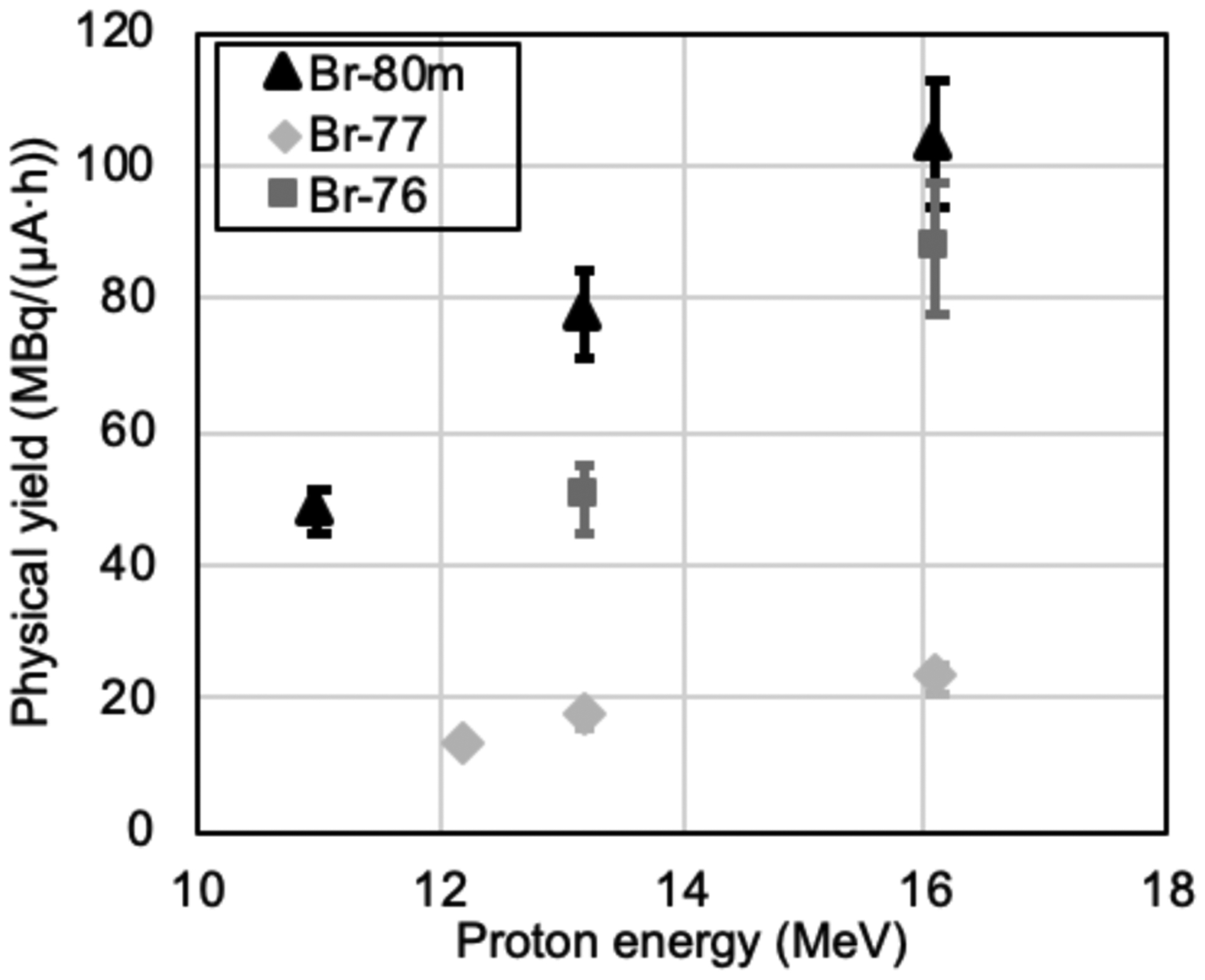
Production yield of 80mBr, 77Br, and 76Br from irradiation of Co80Se, Co77Se, and Co76Se, respectively. Error bars represent standard deviations of measurements from multiple irradiations (see Table 1 for details).
Table 2.
End of bombardment (EoB) radionuclidic purity of 80mBr, 77Br, and 76Br produced at various proton energies (Ep).
| Ep (MeV) | Target | EoB radionuclidic purity | |
|---|---|---|---|
| 16 | Co80Se | 80mBr | |
| Co77Se | 77Br | ||
| Co76Se | 76Br | ||
| 13 | Co80Se | 80mBr | |
| Co77Se | 77Br | ||
| Co76Se | 76Br | ||
| 12 | Co77Se | 77Br | |
| 11 | Co80Se | 80mBr | |
Radiochemical isolation of radiobromine
Thermochromatographic distillation of radiobromine readily occurred within 5 – 10 minutes in a 1050 °C furnace. Typical traces from detectors collimated on the CoSe (Fig. 1, left) and H2O trap (Fig. 1, right) are shown in Figure 4 with detailed explanation in the supplementary material. 96 ± 4% (n=8) of the QMA-loaded 76/77/80mBr was recovered in the K2SO4/NH4OH eluant. Optimized yields of the combined dry distillation and radiobromide recovery process were 76 ± 11% (n=6). The CoSe cyclotron targets lost 0.9 ± 0.5% (n = 20) of their mass with each irradiation/distillation cycle.
Figure 4.
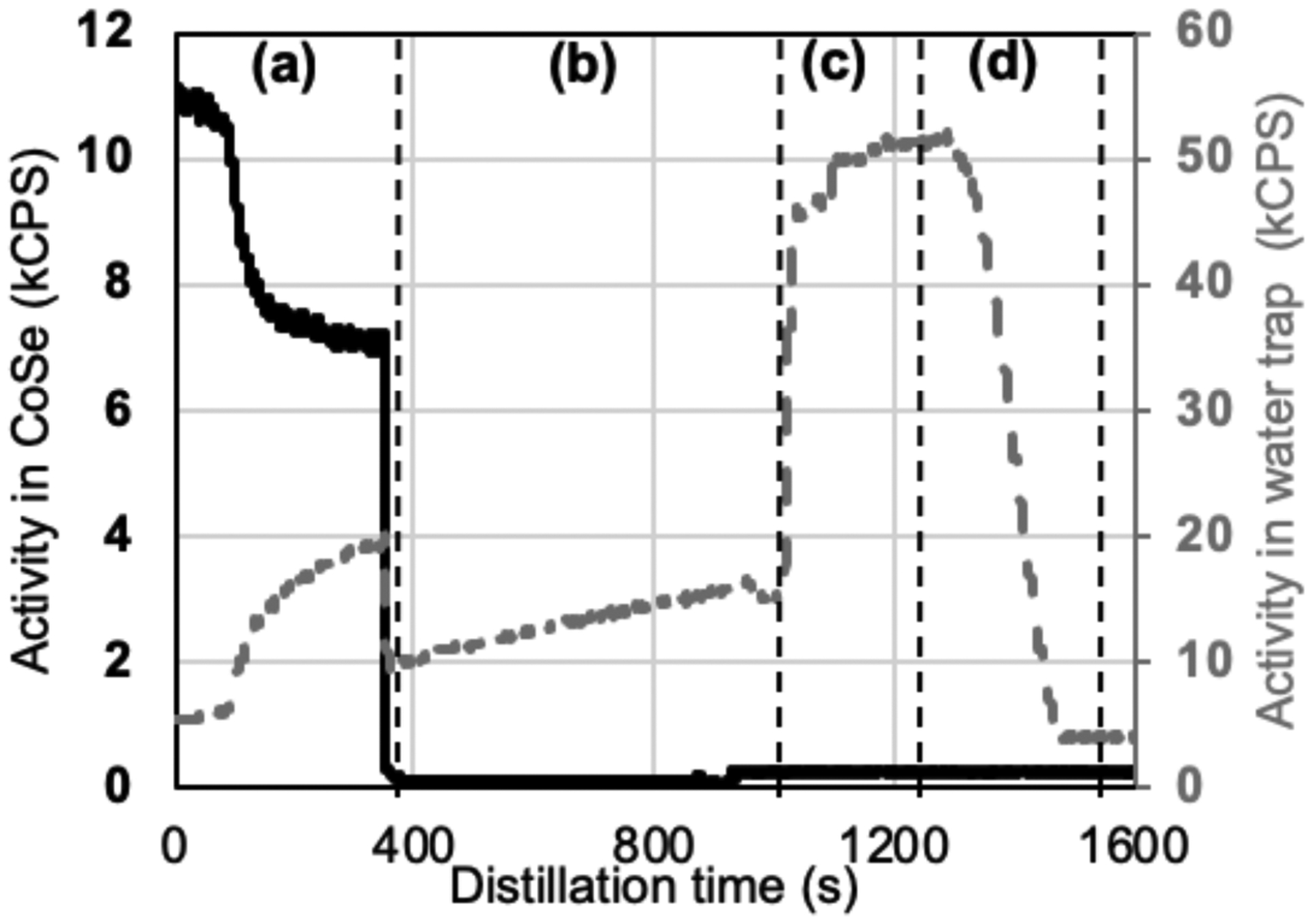
Typical radioactivity profiles in kilocounts per second (kCPS) in the radiobromine distillation assembly. The detector collimated on CoSe (Fig. 1, left) is shown in solid black on the left axis while the detector collimated on the H2O trap (Fig. 1, right) is shown in dashed grey on right axis. Region (a) spans the duration of heating, region (b) spans the quench/cooling period, region (c) spans the H2O rinse of outlet quartz and PTFE lines, and region (d) spans the QMA cartridge loading.
Radiosynthesis of 77Br-PARPi
The [77Br]bromide QMA eluant was either used directly for radiolabeling or after drying under argon flow at 120 °C. Radiochemical conversions from 4.7 – 95% were observed for the reaction conditions, as summarized in Table S3, with conditions of reactions {1–4,6–12} adapted from Reilly et al. [26] and reaction {5} from Zhou et al. [3]. A single radiolabeled peak was eluted from preparative HPLC (see Figure S4), confirmed to be the desired 77Br-PARPi through co-injection with stable iodinated analogue compound (KX1). Based on HPLC absorbance measurements of KX1, the synthesized 77Br-PARPi had an estimated molar activity of up to 700 GBq/μmol (19 Ci/μmol) at the time of analysis.
DISCUSSION
The Co-Se binary phase diagram shows that there exists an intermetallic species with stoichiometric flexibility near Co0.88Se with a melting point of 1078 °C [30]. Described high temperature CoSe preparation methods successfully form this compound (Figure S2) [31] and are significantly faster than multi-step, low temperature (125 – 530 °C) Cu2Se sintering methods [11]. Final CoSe cyclotron targets were energetically “thick” to effectively maximize the production yield of (p,n) nuclear reactions from 13, 12, and 11 MeV protons, but “thin” to 16 MeV protons.
The CoSe targets tolerated higher cyclotron beam intensity (≥40 μA) than Cu2Se targets (15 – 20 μA) [11,12]. Hot pressing wets the niobium backing with the molten CoSe intermetallic, establishing excellent thermal contact with the water-cooled backing allowing for effective removal of the deposited proton beam power. Radiobromine from CoSe is radionuclidically pure (see Table 2) and yields are 1.3 – 2 times greater than those from other selenium alloys [12,13] (see Table 1 and Figure 3). The 77Se(p,2n)76Br threshold limits the radionuclidic purity of 77Br above 13.3 MeV. Measured 77Br yields from Co77Se targets were compared with theoretical yields calculated from measured cross sections [32] and found to be 38% of theoretical at 12 MeV, 43% of theoretical at 13 MeV and 70% of theoretical at 16 MeV. This disagreement was shown (see supplementary material, table S2) to result from a mismatch in proton beam spot and target diameters [33]. The degrader foil increases beam spread and therefore lowers radiobromine yield.
The optimized dry distillation process yielded ~75% recovery of cyclotron produced radiobromine and CoSe targets were exceptionally reusable, with ~1% of CoSe mass lost with each production. This is likely due to the metallurgical properties of the CoSe intermetallic, the short time the targets are heated during distillation, and the rapid quenching that prevents hot CoSe from partitioning into less resilient cobalt- and selenium-containing species during cooling.
Large reaction volume and water content negatively affected radiochemical reactivity the copper-mediated aryl boronic ester bromination. Utilization of hot (80°C) dimethylsulfoxide (DMSO) as reaction solvent in {5} improved radiochemical conversion compared with the similar conditions of {2}. The presence of the K2SO4 impeded the reaction, likely by coordinating and deactivating the tetrakis(pyridine)copper (II) triflate catalyst. Copper sulfate is a poor catalyst in copper-mediated [18F]fluorination of boronic acids [34]. Potassium sulfate was included in these reactions as it is an effective, non-basic QMA release agent for [77Br]bromide. Bromination reactions using [77Br]bromide released from QMA cartridges in 0.1 M NH4OH improved radiochemical conversion, as seen in reactions {6–12}. Optimal radiochemical labeling conditions resulted from reacting dried 77Br in 0.1 M NH4OH eluant with 1 μmol pre-KX1-Bpin, 0.5 μmol Cu(py)4(OTf)2, and 0.5 μmol Lig in 70 μL MeOH at room temperature for 1 hour. The measured molar activity of the radiolabeled compound was exceptionally high, amounting to ~35% of the theoretical maximum 77Br molar activity of 2000 GBq/μmol (55 Ci/μmol).
CONCLUSION
This work presents new methods for cyclotron production and radiochemical isolation of theranostic radionuclides of bromine, including 77Br, 76Br, and 80mBr. Novel accelerator targets of the intermetallic compound CoSe tolerate higher intensity proton irradiations and produce 77Br at three times the rate of previously reported methods. Radiobromine is isolated using a vertical dry distillation assembly that offers several key advantages over horizontal assemblies, including better hot cell compatibility, more rapid heating, and quench cooling of CoSe targets during fabrication and distillation. CoSe targets are resilient to the irradiation/distillation process and individual targets have been reused in 20+ radiobromine productions. Produced [77Br]bromide is radiochemically reactive and has been used to synthesize 76/77Br-based theranostic radiopharmaceuticals with high apparent molar activities.
Supplementary Material
Acknowledgements
This work was supported by United States Department of Energy Office of Science grants DE-SC0017919 and DE-SC0017912. The authors gratefully acknowledge use of facilities and instrumentation at the UW-Madison Wisconsin Centers for Nanoscale Technology (wcnt.wisc.edu) partially supported by the NSF through the University of Wisconsin Materials Research Science and Engineering Center (DMR-1720415).
Footnotes
Disclosure
No potential conflicts of interest relevant to this article exist.
References
- 1.Zhou D, Zhou H, Jenks CC, Lewis JS, Katzenellenbogen JA, Welch MJ. Bromination from the Macroscopic Level to the Tracer Radiochemical Level: 76Br Radiolabeling of Aromatic Compounds via Electrophilic Substitution. Bioconjug Chem 2009; 20:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D, Kim SH, Chu W, Voller T, Katzenellenbogen JA. Evaluation of aromatic radiobromination by nucleophilic substitution using diaryliodonium salt precursors. J Label Compd Radiopharm 2017; 60:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou D, Chu W, Voller T, Katzenellenbogen JA. Copper-mediated nucleophilic radiobromination of aryl boron precursors: Convenient preparation of a radiobrominated PARP-1 inhibitor. Tetrahedron Lett 2018; 59:1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassis AI, Adelstein JS. Lethality of Auger Electrons from the Decay of Bromine-77 in the DNA of Mammalian Cells. Rad Res 1982; 90(2):362–373. [PubMed] [Google Scholar]
- 5.Kao C-HK, Waki A, Sassaman MB, Jagoda EM, Szajek LP, Ravasi L, et al. Evaluation of [76Br]FBAU 3’,5’-dibenzoate as a lipophilic prodrug for brain imaging. Nucl Med Biol 2002; 29:527–535. [DOI] [PubMed] [Google Scholar]
- 6.Katzenellenbogen JA, McElvany KD, Senderoff SG, Carlson KE, Landvatter SW, Welch MJ. 16α-[77Br]Bromo-11β-methoxyestradiol-17β: A Gamma-Emitting Estrogen Imaging Agent with High Uptake and Retention by Target Organs. J Nucl Med 1982; 23(5):411–419. [PubMed] [Google Scholar]
- 7.Lang L, Li W, Jia H-M, Jia H-M, Fang D-C, Zhang S, et al. New Methods for Labeling RGD Peptides with Bromine-76. Theranostics 2011; 1:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mume E, Orlova A, Malmström P-U, Lundqvist H, Sjöberg S, Tolmachev V, Radiobromination of humanized anti-HER2 monoclonal antibody trastuzumab using N-succinimidyl 5-bromo-3-pyridinecarboxylate, a potential label for immunoPET. Nucl Med Biol 2005; 32:613–622. [DOI] [PubMed] [Google Scholar]
- 9.Rowland DJ, McCarthy TJ, and Welch MJ. Radiobromine for imaging and therapy. In: Welch MJ and Redvanly CS, editors. Handbook of Radiopharmaceuticals. New York, NY: John Wiley & Sons Ltd.; 2003, p. 441–465. [Google Scholar]
- 10.Vaalburg W, Paans AMJ, Terpstra JW, Wiegman T, Dekens K, Rijskamp A, et al. Fast Recovery by Dry Distillation of 75Br Induced in Reusable Metal Selenide Targets via the 76Se(p,2n)75Br Reaction. Int J Appl Radiat Isot 1985; 36(12): 961–964. [Google Scholar]
- 11.Tolmachev V, Loevqvist A, Einarsson L, Schultz J and Lundqvist H. Production of 76Br by a Low-energy Cyclotron. Appl Radiat Isot 1998; 49(12):1537–1540. [DOI] [PubMed] [Google Scholar]
- 12.Tang L Radionuclide production and yields at Washington University School of Medicine. Q J Nucl Med Mol Imaging 2008; 52:121–133. [PubMed] [Google Scholar]
- 13.Breunig K, Spahn I, Spellerberg S, Coenen HH. Production of no-carrier-added radiobromine: new nickel selenide target and optimized separation by dry distillation. Radiochim Acta. 2015; 103:397–402. [Google Scholar]
- 14.Ellison PA, Graves SA, Murali D, DeJesus OT, Barnhart TE, Thomadsen BR, et al. Radiobromine Production, Isolation and Radiosynthesis for the Development of a Novel Prostate Cancer Radiotherapeutic Agent. AIP Conf Proc 2017; 1845:020007. [Google Scholar]
- 15.Hassan HE, El-Azony KM, Azzam A, Qaim SM. Investigation of selenium compounds as targets for 76,77Br production using protons of energies up to 34 MeV. Radiochim Acta. 2017; 105(10):841–850. [Google Scholar]
- 16.Qaim SM, Hohn A, Bastian Th, El-Azoney KM, Blessing G, Spellerberg S, et al. Some optimisation studies relevant to the production of high-purity 124I and 120gI at a small-sized cyclotron. Appl Radiat Isot 2003; 58(1):69–78. [DOI] [PubMed] [Google Scholar]
- 17.Nye JA, Avila-Rodriguez MA, Nickles RJ. A new binary compound for the production of 124I via the 124Te(p,n)124I reaction. Appl Radiat Isot. 2007; 65(4):407–412. [DOI] [PubMed] [Google Scholar]
- 18.Aneheim E, Albertsson P, Bäck T, Jensen H, Palm S, Lindegren S. Automated astatination of biomolecules – a stepping stone towards multicenter clinical trials. Sci Rep. 2015; 5:12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalutsky MR, Zhao X-G, Alston KL, Bigner D. High-Level Production of α-Particle-Emitting 211At and Preparation of 211At-Labeled Antibodies for Clinical Use. J Nucl Med 2001; 42:1508–1515. [PubMed] [Google Scholar]
- 20.Wilbur DS, Vessella RL, Stray JE, Goffe DK, Blouke KA, Atcher RW. Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab’)2. In vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab’)2. Nucl Med Biol. 1993; 20:917–927. [DOI] [PubMed] [Google Scholar]
- 21.Freifelder R, Kachur A, LeGeyt BC, Schmitz A, Toto LC. Production of 211At using the JSW BC3015 at the University of Pennsylvania. AIP Conf Proc. 2012; 1509:129–134. [Google Scholar]
- 22.Christian BT, Nickles RJ, Stone CK, Mulnix TL, Clark J. Improving the Radionuclidic Purity of Tc-94m for PET Imaging. Appl Radiat Isot 1995; 46(2):69–73. [Google Scholar]
- 23.Carney B, Kossatz S, Reiner T. Molecular Imaging of PARP. J Nucl Med 2017; 58:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight JC, Koustoulidou S, Cornelissen B. Imaging the DNA damage response with PET and SPECT. Eur. J Nucl Med Mol Imaging 2017; 44:1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanism of action. Sci Trans Med. 2016; 8:362spi17. [DOI] [PubMed] [Google Scholar]
- 26.Reilly SW, Makvandi M, Xu K, Mach RH. Rapid Cu-Catalyzed [211At]Astatination and [125I]Iodination of Boronic Esters at Room Temperature. Org Lett 2018; 20:1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler JF, Ziegler MD, Biersack JP. SRIM - The stopping and range of ions in matter. Nucl Inst Meth Phys Res B, 2010; 268(11–12):1818–1823. [Google Scholar]
- 28.Otuka N, Takács S. Definitions of radioisotope thick target yields. Radiochim. Acta, 2015; 103(1):1–6. [Google Scholar]
- 29.Aničin IV, Yap CT. New approach to detection limit determination in spectroscopy. Nucl Inst Meth Phys Res A. 1987; 259:525–528. [Google Scholar]
- 30.Okamoto H Co-Se (Cobalt-Selenium). In: Massalski TB, editor, Binary Alloy Phase Diagrams vol. 2, Second Edition, Materials Park, OH: ASM International; 1990, p. 1235–1237. [Google Scholar]
- 31.Zhan JH, Yang XG, Li SD, Xie Y, Yu WC, Qian YTJ. Synthesis of Nanocrystalline Cobalt Selenide in Nonaqueous Solvent. Solid State Chem 2000; 152:537–539. [Google Scholar]
- 32.Hassan HE, Qaim SM, Shubin Yu, Azzam A, Morsy M, Coenen HH. Experimental studies and nuclear model calculations on proton-induced reactions on natSe, 76Se and 77Se with particular reference to the production of the medically interesting radionuclides 76Br and 77Br. Appl Radiat Isot 2004; 60:899–909. [DOI] [PubMed] [Google Scholar]
- 33.Hermanne A, Ignatyuk AV, Capote R, Carlson BV, Engle JW, Kellett MA, et al. Reference Cross Sections for Charged-particle Monitor Reactions. Nucl Data Sheets 2018; 148:338–382. [Google Scholar]
- 34.Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, et al. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org Lett. 2015; 17:5780–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


