Abstract
Introduction
Adolescent pregnancy is a known health risk to mother and child. Statements and reports of health outcomes typically group mothers under 20 years old together. Few studies examined this risk at a finer age resolution, none of them comprehensively, and with differing results.
Methods
We analysed Demographic and Health Surveys data from 2004 to 2018 in sub-Saharan Africa (SSA) and South Asia, on firstborn children of mothers 25 years old or younger. We examined the association between maternal age and stillbirths, and neonatal mortality rate (NNMR), infant mortality rate (IMR) and under-5 mortality rate (U5MR), using mixed-effects logistic regression adjusting for major demographic variables and exploring the impact of maternal health-seeking.
Results
In both regions and across all endpoints, mortality rates of children born to mothers aged <16 years, 16–17 years and 18–19 years at first birth were about 2–4 times, 1.5–2 times and 1.2–1.5 times higher, respectively, than among firstborn children of mothers aged 23–25. Absolute mortality rates declined over time, but the age gradient remained similar across time periods and regions. Adjusting for rural/urban residence and maternal education, in SSA in 2014–2018 having a <16-year-old mother was associated with ORs of 3.71 (95% CI: 2.50 to 5.51) for stillbirth, 1.92 (1.60–2.30) for NNMR, 2.13 (1.85–2.46) for IMR and 2.39 (2.13–2.68) for U5MR, compared with having a mother aged 23–25. In South Asia, in 2014–2018 ORs were 5.12 (2.85–9.20) for stillbirth, 2.46 (2.03–2.97) for NNMR, 2.62 (2.22–3.08) for IMR and 2.59 (2.22–3.03) for U5MR. Part of the effect on NNMR and IMR may be mediated by a lower maternal health-seeking rate.
Conclusions
Adolescent pregnancy is associated with dramatically worse child survival and mitigated by health-seeking behaviour, likely reflecting a combination of biological and social factors. Refining maternal age reporting will avoid masking the increased risk to children born to very young adolescent mothers. Collection of additional biological and social data may better reveal mediators of this relationship. Targeted intervention strategies to reduce unintended pregnancy at earlier ages may also improve child survival.
Keywords: child health, public health, descriptive study, maternal health
WHAT IS ALREADY KNOWN ON THIS TOPIC
The under-20 mothers in most previous studies are treated as a single group when looking at risk of child health outcomes, and few studies have assessed the risk gradient versus age within this group, focusing only on neonatal and infant mortality rather than broader child survival outcomes.
WHAT THIS STUDY ADDS
Our work, the most comprehensive, multiregional study to-date, investigated the potential impacts of adolescent pregnancy, examining multiple child survival endpoints from stillbirths to under-5 mortality.
Children of mothers younger than 16 faced 2–4 times higher risk of death at all child mortality stages in both sub-Saharan Africa and South Asia regions, even after controlling for maternal education and health-seeking risk factors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Our findings provide evidence on the necessity for age-disaggregated reporting and additional survey data on adolescent pregnancy, given the specific biological and social risks to adolescents.
Improving health-seeking behaviour and quality of maternal care, as well as targeted interventions to reduce unintended adolescent pregnancy and mitigate its harmful consequences are needed.
Introduction
Every year, nearly 12 million adolescent girls and young women aged 15–19 years and nearly a million under 15 years give birth.1 The majority of these births are in low-income and middle-income countries (LMICs).2 The adolescent fertility rate (birth rate per 1000 girls and young women aged 15–19 years) over the period 2015–2020 was the highest in the sub-Saharan Africa (SSA) region at 102.8 births per 1000 person-years, far higher than the global average (44 per 1000), followed by South Asia with 26 births per 1000 girls aged 15–19.3
Adolescence is a unique stage of human development and an important time for building the foundation of good health; consequently, pregnancy during this lifestage can have impacts on both a young woman and her children. Early pregnancy can lead to devastating health consequences for the mother, since adolescent girls may not yet be physically and biologically ready for pregnancy or childbirth.3 Many adolescents experience complications during pregnancy and childbirth, which has become the leading global cause of death among 15–19-year-old females.4 Pregnant adolescents are at a higher risk of receiving inadequate antenatal care (ANC) in some settings.5 A significant proportion of adolescents in SSA do not access nor utilise maternal services during pregnancy, which is a consequence of several individual, interpersonal, institutional and systemic factors.6 Early pregnancy and motherhood for an adolescent girl in some contexts can also have adverse social consequences such as stigma and dropping out of school.1 7 They may not have the opportunity to return to school which jeopardises their economic and employment opportunities due to their double burden of household maintenance and child-rearing,7 8 resulting in sustained poverty and increased vulnerability.
Reduction of adolescent pregnancy has long been the focus of several organisations and is of current policy interest. In fact, with only 8 years left to achieve the 2030 Agenda for Sustainable Development, agreed to by more than 190 countries, there remains a timely commitment and need to ensure access to sexual and reproductive healthcare services, particularly for adolescent girls and young women (Target 3.7), and eliminate child, early and forced marriage (Target 5.3), given their strong associations with adolescent pregnancy and its outcomes.9 Despite these efforts and the recent decline in overall adolescent mortality10 and global adolescent fertility rate, prevalence of adolescent pregnancies remains high and a major public health concern, especially in LMICs.
In standard surveys, reports and WHO statements, mothers under 20 are usually treated as a single group.11 However, adolescence represents a time of developmental transition, including physically, cognitively and psychologically, and there are substantial differences across the 10–19 years age range.12 Few studies have looked at the risk gradient versus age among young mothers. Several studies have associated early maternal age with neonatal and infant mortality,2 9 13 14 infant stunting and preterm birth even after adjustment for sociodemographic factors.15 In contrast, two recent multicountry studies did not find a consistent significant association between adolescent motherhood and stillbirth.16 17 Current findings and studies leave unanswered questions about the true nature of these relationships.
A meta-analysis of Demographic and Health Surveys (DHS) showed higher risk of mortality to neonates born to mothers aged <16 and 16–17 years old than neonates born to mothers aged 20–29 years in SSA and South and Southeast Asia,11 even after adjusting for socioeconomic, demographic and health service utilisation variables. In LMICs, the infant mortality rate was higher among mothers with ages of 12–14 and 15–17 years than among older mothers.13 Finlay et al14 showed in a separate analysis that the risk of infant mortality in SSA is highest for high parity young mothers, and short birth intervals negatively affect infant mortality and stunting outcomes. A WHO multicountry study divided mother ages into <16, 16–17, 18–19 and 20–24 years old. They found stillbirth rates among adolescent mothers to be mildly higher than 20–24 years old mothers (ORs 1.0–1.3), with the difference significant only for the 16–17 years old group.17 A more recent study examined the association between maternal age, both young and advanced and risk of neonatal mortality in LMICs using DHS data, and found the risk of mortality of neonates born to mothers aged 12–15 and 45+ years was higher than neonates born to mothers aged 25–29 years.18 A systematic review and meta-analysis in SSA found that most evidence about the effects of early childbearing was for mothers 15–19 years old as a single group, with very few studies providing data on adolescents aged <18, and concluded that there is a lack of high-quality observational studies that adjust for sociodemographic factors.19 Overall, there are limited number of studies focusing on risk gradient versus maternal age among young mothers, and majority of these studies focused on neonatal and infant mortality rather than broader child survival outcomes.
In our study, the most comprehensive of its kind to date, we have investigated the potential impacts of adolescent pregnancy on a substantially broader scope than previous studies, examining child mortality endpoints from stillbirths to under-5 mortality, and quantifying the risk gradient as a function of age from adolescence through young adulthood. In contrast to prior studies which focused mostly on survival endpoints around birth, we hypothesised that since adolescent mothers face greater physical, emotional and social challenges, the impact on their offspring’s survival might be felt throughout early childhood. In addition, to examine whether observed associations between maternal age and child survival may be caused by confounding variables that affect both, we explored adjustment for key demographic variables such as urban versus rural residence. We also investigated whether the association between mother’s age and child mortality endpoints might be mediated by maternal health-seeking. We focused on SSA, the region with the highest adolescent pregnancy and child mortality burdens, as well as South Asia, the second-highest region in child mortality burden where adolescent pregnancy rates fell rapidly in recent years. The comprehensiveness and multiregionality of our analysis helps frame the inconsistent findings from previous studies9 13–17 on the relationship between maternal age and child health outcomes. Disaggregation of the adolescent age group helps highlight the increased risk of younger adolescents and the potential benefits of providing health services for these girls.
Methods
Data source and study population
We analysed DHS data collected between 2004 and 2018 from countries in SSA and South Asia. DHS are cross-sectional nationally representative large-scale household surveys that collect and analyse demographic, health and nutrition data, in a manner that enables comparisons across countries and over time.20 The women’s questionnaire, used in this study, invites all women aged 15–49 in a surveyed household to respond. In a few surveys, the target group was women and girls aged 10–49 years old. We defined three time periods: 2004–2008, 2009–2013 and 2014–2018, to assess the variation in each outcome over time. We estimated the risk gradient versus age at first birth among adolescent and young adult mothers. We considered only first births in order to avoid the various confounders associated with parity, and also because most adolescent births are first births. Since the vast majority of first births in the two regions take place by women’s mid-20s, we restricted the analysis to mothers 25 years old or younger. In total, 35 countries with 80 surveys in SSA and 11 countries with 27 surveys in South Asia were included in the analysis (online supplemental material).
bmjgh-2021-007681supp001.pdf (5.1MB, pdf)
Endpoints and risk factors
Maternal age at first birth within the 10 years preceding the survey was divided into five groups: <16, 16–17, 18–19, 20–22 and 23–25 years old. For stillbirth, DHS only collects data from the 5 years preceding the survey. The age group <16 includes mothers aged 10–15 years old, or 15 years old in surveys restricted to women aged 15–49. Risk factors accounted for in this analysis are sociodemographic factors: urban/rural residency and maternal education status (dichotomised as any education vs none); economic factors: wealth quintile (a country-specific measure of the household wealth compared with other households in each survey and grouped as poorest, poorer/middle/richer and richest in our analysis); and health-seeking factors: place of delivery (at home vs health facility) and ANC utilisation (no ANC visit vs any ANC visit). When we used literacy instead of education, we found similar results and thus we did not include it in our analysis. All risk factors were coded as categorical variables in our analysis.
We examined the following outcomes in our study: stillbirth (pregnancies that lasted 7 or more months and terminated in fetal death), neonatal mortality (death after a live birth within the first 28 days of life), infant mortality (death within the first year of life), child mortality (death after the first year and before reaching the age of 5 years), 1–59 month mortality (death after the first month and before reaching the age of 5 years) and under-5 mortality (death before reaching the age of 5 years). Of these, stillbirths, neonatal, infant and under-5 mortality are reported in the main article, and the remaining endpoints in online supplemental material.
Statistical analysis
Descriptive analyses included calculating the neonatal, infant, child and under-5 and 1–59 month mortality rates per 1000 live births and their sampling errors based on the DHS mortality rates estimation methodology, a synthetic cohort life table.21 We calculated the mortality rates for three time periods in each region and for each maternal age group combined with selected risk factors. Among multiple births (twins, triplets, etc), only those with the birth assigned order number of 1 by DHS were considered in the analysis. While excluding the remaining multiple-birth siblings reduces the sample size by about 1%, it helps simplify and stabilise the analysis by avoiding the need to account for another level of dependence.
A mixed-effect logistic regression model was applied to each time period and each region separately, to examine the association between each of the outcome variables and the risk factors. The sample size for the maternal age group and sociodemographic factors are the same and therefore, these models are layered stepwise and comparable. A model including only age group and the random effects was developed, called here Model 0. The model was further adjusted for different combinations of risk factors, based on prior knowledge of common risk factors for LMIC child mortality, as well as to assess the impact of leading healthcare access and socioeconomic indicators on the risk gradient. In Model 1, maternal age at first birth, urban/rural residency and maternal education status were included. Questions about place of delivery and ANC utilisation were available for the last births in the 3/5 years preceding the survey. A model adjusting for maternal age and healthcare accessibility factors was developed, called here Model 2. All the first births that happened within the 3/5 years preceding the survey were included in Model 2. Given that the primary sampling units, year of survey and country name were used as nested random effects in the model, the wealth index represents the deviation of household wealth from its own country’s mean wealth at the time of interview11 and Model 1 was further adjusted for the wealth index, called here Model 3. We also combined the two healthcare variables into one and grouped the outcomes as having either ANC visit or facility birth; both ANC visit and facility birth; and no ANC visit and home delivery, and further adjusted Model 1 for this variable, called here Model 4. Note that despite including Model 1’s variables, Model 4 is not nested within Model 1 as reported here, because the health-seeking variables were only available for each mother’s last birth. Together with the first-birth constraint of all models, this reduces the sample size by 5–7 times, as well as weights the sample more towards recent first births. Models 0–2 are reported in the main article, and Models 3 and 4 in the online supplemental material. Models 2 and 4 are layered stepwise and comparable, separately from Models 0, 1 and 3. Each survey’s sample weights were used as the model prior weights in the fitting process. Mothers of age 23–25 years old were considered the reference age group. All the analyses were performed in R V.4.0.22
Patient and public involvement statement
Study participants or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Univariate maternal-age risk gradient
The numbers of first births and neonatal, infant, child and under-5 deaths to women aged under 25 years old that occurred within 10 years preceding the survey, grouped by region, survey period, and age, are given in online supplemental tables 1 and 2. Within each time period, about 20%–23% of first births to mothers 25 years old or younger in SSA were attributed to mothers under 18 years old. In South Asia, this proportion was lower and decreased from 15.7% in 2004–2008 to 8.1% in 2014–2018.
The majority of women across all ages and time periods lived in rural areas. About 60% and 43% of mothers aged <16 years old, respectively, in SSA and South Asia, in the 2004–2008 period had no formal education. This rate decreased with age and time in SSA, but a similar trend was not observed in survey data from South Asia (online supplemental table 2). In both regions, about 80% of women had at least one ANC visit. About 45%–48% of pregnant women aged <16 years old in both regions gave birth at home during the 2014–2018 period, two to three times more often than mothers over 20 years old. The gap has not narrowed substantially between 2004–2008 and 2014–2018 (online supplemental tables 1 and 2).
The mortality rates for different child outcomes and their sampling errors are given in table 1 for SSA and South Asia and online supplemental tables 3 and 4, respectively. In SSA, neonates, infants and children under-5 born to mothers aged <16 were at about two times higher risk of death (54.5±4.5, 95.9±5.5 and 156.5±6.6 deaths per 1000 live births, respectively, in 2014–2018) than those born to mothers aged 23–25 years old (28±1.6, 44.5±1.9 and 64.8±2.4, respectively). The mortality rates for children of the <16 age group in South Asia were two to three times higher (59.7±15, 84.4±20.8 and 95.7±22.8, respectively) than the oldest age group (26.3±1.6, 34.4±3.3 and 40.7±3.3, respectively), however the uncertainty intervals are wide for the youngest age group. Overall, mortality rates decreased with time, but the risk gradient versus age has remained similar.
Table 1.
Mortality rates of different child health outcomes and their sampling errors in sub-Saharan Africa and South Asia
| 2004–2008 | 2009–2013 | 2014–2018 | |||||||
| NNMR | IMR | U5MR | NNMR | IMR | U5MR | NNMR | IMR | U5MR | |
| Sub-Saharan Africa | |||||||||
| Maternal age at first birth (years) | |||||||||
| <16 | 85.1±5 | 152.8±6.5 | 238.5±7.5 | 63.8±4.0 | 117.7±5.3 | 186.2±6.2 | 54.5±4.5 | 95.9±5.5 | 156.5±6.6 |
| 16–17 | 58.3±2.3 | 115.8±3.1 | 188.3±3.9 | 49.8±1.9 | 91.3±2.6 | 145.2±3.3 | 44.4±2.0 | 72.5±2.6 | 112.8±3.2 |
| 18–19 | 46.9±1.8 | 89±2.3 | 146±2.9 | 39.1±1.4 | 73.8±1.9 | 115.5±2.3 | 34.4±1.4 | 58±1.8 | 88.2±2.2 |
| 20–22 | 39.9±1.4 | 79.3±2.1 | 127.3±2.6 | 35.8±1.2 | 62.4±1.6 | 98.6±2.0 | 29.8±1.2 | 50.3±1.5 | 74.6±1.8 |
| 23–25 | 40.2±2.1 | 74.5±2.8 | 123.5±3.7 | 33.7±1.8 | 55.7±2.2 | 88.8±2.7 | 28±1.6 | 44.5±1.9 | 64.8±2.4 |
| Urban/rural residency | |||||||||
| <16 | 53.2±2.9 | 115.6±6.1 | 182.6±10 | 58.3±16.2 | 108.1±16.2 | 159.1±15.5 | 43.2±3.1 | 74.1±9 | 115.9±10.6 |
| 92.6±13.7 | 161.6±13.1 | 251.9±10.5 | 65.8±1.0 | 121.3±1.9 | 196.1±3.9 | 58.6±18.7 | 103.6±18.3 | 170.9±16.8 | |
| 16–17 | 50.2±2.4 | 97.6±2.3 | 160.4±2.3 | 30.2±3.2 | 63.7±3.2 | 100.1±3.4 | 39±13.2 | 62.4±13.9 | 90.6±13.6 |
| 60.7±2.1 | 121.2±3.1 | 196.8±6.9 | 57.8±2.0 | 102.5±2.3 | 163.6±2.8 | 46.3±1 | 76.2±1.7 | 121.2±2.3 | |
| 18–19 | 39.2±2.4 | 75.2±2.5 | 119.2±2.9 | 33.5±1.8 | 59.9±2.0 | 87.8±2.7 | 25.9±3.2 | 45.2±4 | 67.5±4.1 |
| 49.8±2.7 | 94.1±2.9 | 156±3.4 | 41.6±2.0 | 80.3±2.8 | 128.4±3.6 | 37.9±3.1 | 63.5±3 | 97.1±3 | |
| 20–22 | 30±1.8 | 60.6±1.8 | 95.2±1.9 | 30.7±0.3 | 49.5±0.7 | 72.9±1.3 | 25.5±0.9 | 41.3±1.5 | 57.9±4.5 |
| 44.2±1.2 | 87.4±1.3 | 141.4±1.8 | 38.7±1.4 | 69.6±3.3 | 113.0±4.5 | 32.2±1.4 | 55.4±1.5 | 84±1.5 | |
| 23–25 | 30.2±0.5 | 56.9±2 | 83.3±2.1 | 32.4±0.3 | 47.9±0.9 | 72.4±1.2 | 27.6±0.4 | 40.5±0.5 | 55.4±0.8 |
| 46.4±1.3 | 85.4±2.3 | 147.3±4.4 | 34.7±4.3 | 62.0±4.1 | 101.7±4.8 | 28.4±0.2 | 48±0.4 | 72.8±0.6 | |
| Maternal education status: any education/no education | |||||||||
| <16 | 69.5±0.9 | 124.7±1.6 | 185.3±1.8 | 59.5±12.8 | 112.7±12.2 | 169.8±11.7 | 55.4±10.8 | 93±11.9 | 140.4±12.1 |
| 95.2±15.7 | 170.8±15.7 | 271.5±13.8 | 67.6±1.3 | 122.1±3.4 | 199.1±5.3 | 53.4±16.8 | 99.2±17.3 | 174.5±15.8 | |
| 16–17 | 51.6±0.4 | 98.6±0.8 | 150.2±1.2 | 42.1±0.4 | 77.4±0.8 | 121.6±1.3 | 40.8±8.7 | 67.3±9.1 | 97.7±8.8 |
| 64.9±2.8 | 132.6±5.1 | 224.7±9.4 | 60.3±3 | 110±3.3 | 174.7±3.6 | 50.6±0.7 | 81.4±1.1 | 136.3±1.7 | |
| 18–19 | 37.7±2.2 | 74.3±2.1 | 117.8±2.2 | 35±0.3 | 65.9±2.3 | 98.5±3.6 | 31.5±2 | 52.3±2 | 76.4±2.3 |
| 61.6±4.5 | 112.1±4 | 189±4 | 46.4±3.8 | 87.8±5 | 143.3±5.4 | 41.1±6.7 | 71.2±6.4 | 113.4±6.4 | |
| 20–22 | 31.8±0.2 | 64.3±0.4 | 100.8±0.6 | 32.5±0.3 | 56.2±0.5 | 84.3±1.9 | 27.8±0.7 | 45.1±1.1 | 65.1±1.6 |
| 56.4±2.4 | 109.2±2.4 | 178.7±3.6 | 42.2±2.6 | 74.4±5.8 | 124.2±7.6 | 35.1±3 | 64.2±2.8 | 97.9±2.6 | |
| 23–25 | 35±0.4 | 65.1±0.7 | 102.1±0.9 | 32.1±0.2 | 51.1±0.4 | 78±0.5 | 28.1±0.3 | 43±0.5 | 58.7±0.7 |
| 52.3±2.3 | 96.3±4.2 | 169.9±7.5 | 37.1±6.7 | 65.2±6.5 | 109.6±7.6 | 27.9±0.1 | 48.9±0.1 | 81.1±0.2 | |
| Antenatal care visit*: any visit/no visit | |||||||||
| <16 | 33.5±1.6 | 79.9±4 | 187.4±15 | 26.7±18.6 | 60.7±22.3 | 109.3±30.8 | 27.9±3.6 | 43.9±5.7 | – |
| 38.5±3.6 | 53.2±5 | 141.3±16.2 | 81.9±16.9 | 122.1±20.7 | 209.1±47 | 26.1±1.8 | 85.2±17.6 | ||
| 16–17 | 27±1 | 60.2±2.2 | 94.2±11.1 | 24.8±0.5 | 51.6±1.1 | 93±2 | 24.3±0.7 | 39.8±1.1 | 64.8±1.3 |
| 57.4±2 | 93.6±10.6 | 173.1±11.8 | 28.8±14.7 | 64.3±14.4 | 95.8±14.2 | 41±1.5 | 79.2±2.8 | 41±5.2 | |
| 18–19 | 25.7±0.7 | 48.5±1.4 | 80.8±2.4 | 23.7±0.4 | 47.8±3.8 | 87.9±3.7 | 19.4±3.7 | 32.1±3.5 | 52.4±3.3 |
| 0.8±1.7 | 77.3±3.3 | 132.6±3.6 | 36.6±1.3 | 60.3±7.6 | 176.2±11.1 | 38.3±1.3 | 62.8±2.3 | 103.9±3.4 | |
| 20–22 | 22.8±0.4 | 48.5±0.7 | 77.3±1.1 | 22.6±0.4 | 41.1±0.6 | 59.8±0.8 | 15.9±0.2 | 27.4±0.3 | 38.2±0.4 |
| 53±2 | 101.7±4 | 206.9±18.9 | 6.2±15.3 | 74.5±18.2 | 150.1±19.7 | 42.8±1.6 | 82.1±2.9 | 125.4±4.2 | |
| 23–25 | 26.1±0.7 | 52.3±1.4 | 82.7±1.9 | 21.3±0.3 | 36.8±0.4 | 65.4±0.5 | 16.5±0.9 | 24.9±1 | 34.1±1.4 |
| 24.7±1.2 | 43.1±15.4 | 132.2±248.3 | 20.6±1.2 | 44.9±2.7 | 63.1±3.8 | 15.2±1.2 | 52.3±4.5 | 96.1±9.9 | |
| Place of delivery*: at home/health facility | |||||||||
| <16 | 88±8.8 | 161±9.5 | 253.1±11.6 | 65.5±2.9 | 116.9±5.3 | 214.3±11.9 | 53±26.4 | 105.7±25.1 | 215.4±22.2 |
| 59.8±19 | 94.2±21.5 | 188.8±31.2 | 38.6±31.1 | 73.6±31.3 | 138.2±29.2 | 41.1±2.1 | 78.6±11 | 140±11.9 | |
| 16–17 | 53.8±1.2 | 108.5±2.5 | 187.3±5.4 | 56.3±1.5 | 90.3±2.5 | 156.1±5.5 | 51.2±1.1 | 80.8±1.7 | 150.9±3.1 |
| 41.7±3 | 88.6±3.9 | 138.1±9 | 32.9±0.6 | 68.1±1.3 | 114.7±2.6 | 34.7±1 | 60.1±1.6 | 91.6±1.8 | |
| 18–19 | 46.5±5.2 | 92.5±5 | 148.6±7.2 | 41.5±8.4 | 78.9±7.7 | 132.1±7 | 41.2±7.5 | 72.5±6.7 | 117±6.1 |
| 35±1.1 | 65.1±2 | 100.9±2.9 | 32.2±0.6 | 59.8±1.1 | 94.4±1.8 | 30.9±0.7 | 46.9±1 | 68.2±1.7 | |
| 20–22 | 43.5±0.7 | 90.3±1.4 | 141.8±2.8 | 44.2±1.3 | 70.8±2.2 | 110.4±4.1 | 38.2±6.7 | 70.6±6.5 | 104.9±6.5 |
| 33.3±0.6 | 65.2±1.1 | 100.7±1.7 | 31.1±0.6 | 53.2±1.1 | 79.6±1.8 | 27.6±0.3 | 43.4±0.4 | 57.9±0.4 | |
| 23–25 | 45.9±4.4 | 83.6±7.1 | 140.6±6.9 | 30.3±0.9 | 60.4±1.7 | 96.7±21.3 | 25.4±0.8 | 48.3±1.6 | 74.5±2.9 |
| 31.7±0.6 | 60.2±1.2 | 97.2±1.7 | 30.7±0.3 | 47.9±0.4 | 75.2±0.4 | 29.5±0.6 | 41.3±0.8 | 53.6±1.1 | |
| South Asia | |||||||||
| Maternal age at first birth (years) | |||||||||
| <16 | 82.9±1.6 | 112.6±2.1 | 145±2.5 | 55.4±2.7 | 81.7±3.1 | 98.9±6.4 | 59.7±15 | 84.4±20.8 | 95.7±22.8 |
| 16–17 | 65.6±2.3 | 94.8±5 | 112.1±4.9 | 50.8±1.1 | 75±2.8 | 86.9±2.9 | 46.4±3.7 | 64.4±5.1 | 74±5.7 |
| 18–19 | 53.2±0.7 | 74.3±1.1 | 86.5±1.2 | 45.7±1.1 | 61.6±1.3 | 71±2.3 | 38.7±7.2 | 51.4±7.3 | 58.7±8.5 |
| 20–22 | 39.6±1.1 | 56.4±1 | 66.9±1 | 32.5±1.5 | 45.7±1.5 | 53.6±1.5 | 31.3±4.6 | 41.9±4.6 | 48.3±4.6 |
| 23–25 | 35.5±0.2 | 49.6±0.3 | 57.5±0.4 | 25.3±0.1 | 36.4±0.3 | 45.1±0.3 | 26.3±1.6 | 34.4±3.3 | 40.7±3.3 |
| Urban/rural residency | |||||||||
| <16 | 80.2±4.2 | 107.3±5.6 | 132.1±6.5 | 48.8±6.2 | 63.5±12 | 86.4±11.8 | 56.4±33.5 | 71.8±42.2 | 77.1±44.1 |
| 83.4±1.6 | 113.6±2.1 | 147.6±2.5 | 57.3±2.8 | 87.1±4.2 | 102.6±7.5 | 60.6±52.8 | 88±51.4 | 101.2±50.5 | |
| 16–17 | 51.1±1.7 | 71.5±2.4 | 87.1±3.2 | 34.4±3.1 | 53.8±4.8 | 60.5±4.8 | 27.3±4.5 | 44.4±7.3 | 54.5±8.6 |
| 69±2.2 | 100.3±4.9 | 118.1±4.7 | 55.7±3.7 | 81.2±4.3 | 94.4±4.4 | 52.8±6.1 | 71.1±6.7 | 80.5±10.1 | |
| 18–19 | 36.5±0.4 | 53.3±0.5 | 61±0.6 | 31±1.6 | 48±2.1 | 52.6±3.4 | 30.4±15.2 | 40.5±15.3 | 45.8±17.8 |
| 58.2±0.8 | 80.7±1.2 | 4.4±1.4 | 50.7±1.6 | 66.1±1.8 | 77.1±2.6 | 41.5±1.9 | 55.1±2.5 | 63.2±2.8 | |
| 20–22 | 28±0.2 | 39.4±0.3 | 45±0.4 | 21.3±0.7 | 30.1±0.7 | 35.1±1 | 23.1±8.8 | 31.2±8.7 | 35.1±8.7 |
| 44.8±1.2 | 64.1±1.1 | 76.8±1 | 37.7±1.7 | 52.9±1.7 | 62.4±1.7 | 34.6±0.9 | 46.3±1.4 | 53.6±1.5 | |
| 23–25 | 27.1±0.2 | 37.2±0.2 | 43.5±0.3 | 19.2±0.1 | 28.7±0.5 | 35.6±0.5 | 16.6±2.5 | 21.9±5 | 26.8±5 |
| 41.4±0.3 | 58.2±0.5 | 67.3±0.6 | 9.4±0.2 | 41.5±0.3 | 51.5±0.3 | 31.9±0.4 | 41.6±0.6 | 48.8±0.7 | |
| Maternal education status: any education/no education | |||||||||
| <16 | 67.4±1.9 | 95±2.7 | 120±3.2 | 42.9±3.2 | 62.7±3.4 | 82.6±7.6 | 67.2±17.9 | 85.7±22.5 | 96±24.3 |
| 100.1±0 | 132±0 | 170.9±0.4 | 100.2±4.6 | 149.4±6.7 | 159.5±6.9 | 45.1±8.3 | 81.5±14.4 | 94.6±16.9 | |
| 16–17 | 52.7±3.9 | 76.8±3.7 | 88.3±3.6 | 40.3±0.9 | 61±3.1 | 68±3.1 | 42.1±3.2 | 57.4±4.4 | 65±4.8 |
| 83.1±0.8 | 118.8±5.2 | 142.5±5 | 94.5±1.6 | 132.8±2.2 | 160.5±2.6 | 56.8±7.1 | 80.8±9.9 | 94.3±11.1 | |
| 18–19 | 44.2±0.7 | 61.6±1.4 | 70.7±1.4 | 39.8±1.1 | 52.6±1.3 | 61.5±2.6 | 35.4±1.3 | 45.7±1.6 | 51.6±6.7 |
| 68.9±0.5 | 96.6±0.7 | 113.2±0.8 | 72.6±0.8 | 102.5±1.1 | 113.6±1.2 | 47.7±20.4 | 66.8±20.5 | 77.4±20.4 | |
| 20–22 | 32.4±1.5 | 46.3±1.4 | 53.5±1. | 28.2±1.7 | 40.3±1.7 | 47.3±1.7 | 28.7±0.9 | 37.2±1.2 | 42±1.3 |
| 59.1±0.2 | 83.8±0.3 | 102.1±2.9 | 57.5±0.3 | 76.7±0.4 | 89.5±0.5 | 39.9±16 | 56.9±15.8 | 67.4±15.7 | |
| 23–25 | 29±0.2 | 39.3±0.3 | 45.7±0.4 | 22.4±0.1 | 31.8±0.4 | 39.3±0.4 | 22.8±1.9 | 28.7±1.9 | 33.8±1.9 |
| 63.8±0.8 | 93.8±1 | 107.2±1 | 46±0.6 | 68.9±1 | 85.4±1.2 | 40.8±2.3 | 57.1±6.4 | 67.5±6.6 | |
| Antenatal care visit: any visit/no visit | |||||||||
| <16 | 23.4±0.8 | 56.3±2 | 67.1±2.4 | – | – | – | 29.2±2 | 29.2±2 | 55.7±7.8 |
| 62.4±5.6 | 63.8±5.7 | 65.8±5.9 | 40.3±30.9 | 40.3±30.9 | 40.3±31 | ||||
| 16–17 | 26.4±0.5 | 38.9±0.7 | 56±1.3 | 19.3±2.1 | 23.7±2.2 | 27.1±7 | 19±2 | 25.9±2.8 | 28.4±3.2 |
| 29.1±0.7 | 65.2±11.9 | 69.8±11.9 | 27.1±1.4 | 32±5.1 | 39.2±5.3 | 39.8±3.2 | 67±5.6 | 83±7.2 | |
| 18–19 | 26.8±0.4 | 35.5±0.5 | 40.3±0.6 | 19.9±0.4 | 24.1±0.4 | 27±0.5 | 17.8±1.2 | 25±1.7 | 27.3±1.9 |
| 25.6±0.4 | 31.6±7.9 | 34.7±7.8 | 20.7±0.8 | 39.6±1.5 | 47.8±1.8 | 38.4±2.7 | 59.4±4.1 | 64.8±4.7 | |
| 20–22 | 23.3±0.2 | 31.4±0.2 | 36.3±0.2 | 12.9±3.6 | 16±3.6 | 17.5±3.6 | 16.6±0.7 | 22.9±0.9 | 25.8±1 |
| 38.2±0.9 | 52.4±1.3 | 75.1±2.1 | 24.1±6.5 | 37.9±6.5 | 39.3±8.3 | 34.6±6 | 46.6±8.1 | 54.7±8.4 | |
| 23–25 | 16.8±0.2 | 22.7±0.2 | 23.2±0.2 | 11.9±0.1 | 18.5±0.7 | 24.9±0.7 | 15.7±3.4 | 20.4±3.4 | 24±3.5 |
| 56.6±3.2 | 81.8±4.5 | 89±5.1 | 39.2±4.3 | 44.9±5 | 44.9±5 | 36±2 | 52±2.9 | 70.2±3 | |
| Place of delivery: at home/health facility | |||||||||
| <16 | 57.3±2.2 | 87.3±3.5 | 120.7±5.1 | 28.1±1.8 | 34.9±2.3 | 50.3±35.8 | 53.7±14.9 | 79.4±24.1 | 112.5±24.2 |
| 74.1±6.1 | 109.3±8.7 | 109.3±8.7 | 40.7±40.4 | 65.7±39.7 | 65.7±39.7 | 55.7±2.6 | 75.3±3.6 | 141.1±9.2 | |
| 16–17 | 61.2±3.3 | 91.9±5.4 | 103.9±5.3 | 36.3±0.8 | 56.2±1.1 | 64.3±1.1 | 53.9±4 | 80.4±5.9 | 88.2±6.1 |
| 62±1.8 | 83.6±2.4 | 94.3±2.6 | 38±0.8 | 38±0.8 | 47.7±1.2 | 41.7±3.5 | 56.9±4.9 | 67.4±6.5 | |
| 18–19 | 50.4±1.2 | 69.9±3.6 | 89±3.5 | 48.5±0.9 | 58.5±1.1 | 68.7±6.3 | 43.4±2 | 64.4±3.2 | 70.1±3.3 |
| 47.2±0.6 | 60±0.7 | 66.8±0.8 | 32.6±0.9 | 40.9±1.2 | 46.2±1.4 | 34.7±3 | 46.5±4.1 | 52±4.7 | |
| 20–22 | 43.4±2.6 | 61±2.5 | 78.1±3.8 | 30.4±0.5 | 41.5±0.7 | 49.7±0.9 | 46±4.7 | 60.9±6.4 | 79.1±10.1 |
| 30±0.3 | 38.3±0.4 | 43.1±0.4 | 26±0.2 | 32.3±0.2 | 38±0.2 | 27.2±1.9 | 37.1±2.6 | 43.7±3 | |
| 23–25 | 46.9±0.6 | 68.2±0.8 | 76.6±0.9 | 29.2±0.5 | 43.5±0.7 | 53.8±0.9 | 42.4±1.5 | 58.1±9.2 | 73.2±9.2 |
| 29.3±0.4 | 36.7±0.5 | 41.8±0.5 | 19.2±0.1 | 27.2±1.4 | 33.2±1.4 | 24.4±3 | 31.3±3.2 | 38.2±3.3 | |
*Births that occurred in the 3/5 years preceding the survey.
IMR, infant mortality rate; NNMR, neonatal mortality rate; U5MR, under-5 mortality rate.
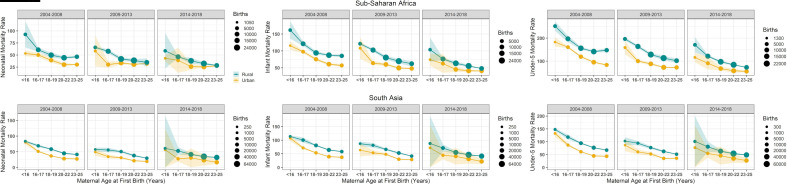
In both regions, the risk gradient versus age appears in both rural and urban locations (figure 1). Similar age gradients were observed when dividing mothers by maternal education status and other variables (table 1).
Figure 1.
Neonatal (left), infant (centre) and under-5 (right) mortality rates and their sampling errors within each age group and urban (yellow) and rural (green) locations in SSA (top) and South Asia (bottom) by 5-year time period: 2004–2008, 2009–2013 and 2014–2018. The circle size represents number of births within each group.
Multivariate analysis adjusting for risk factors
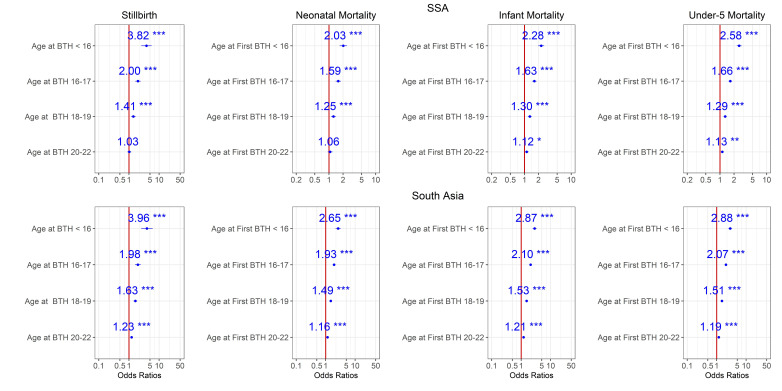
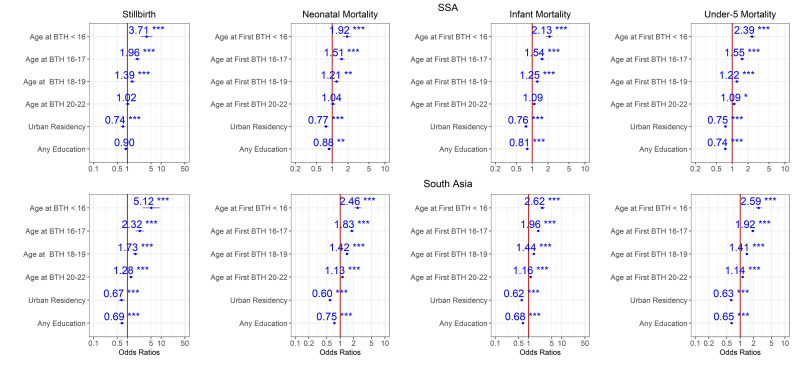
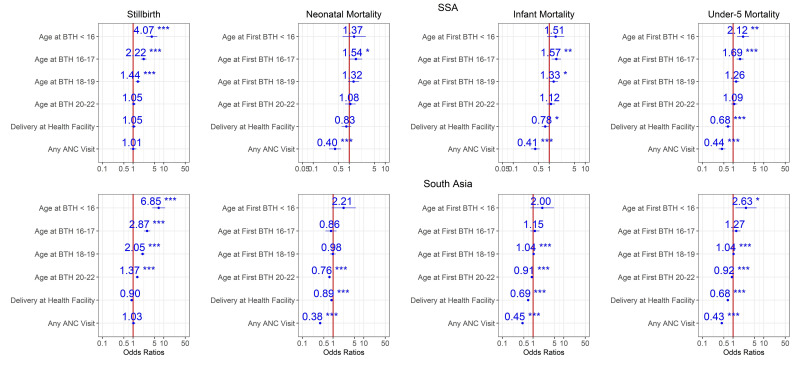
Estimates from Models 0, 1 and 2 for the period 2014–2018 are shown in figures 2–4. According to these models, adjusted maternal-age effects are consistent with the patterns of table 1 and figure 1. Mortality risk for all endpoints increases with younger age, and children in both regions born to mothers aged <16 faced 2–4 times higher mortality risk than those born to mothers aged 23–25 years old at all stages, from stillbirth to under-5 mortality (figure 2), even after adjustment for demographic factors (figure 3). The OR for stillbirth is particularly high with under-16 mothers, around 4 or more in both regions and models. Adjusting for health-seeking variables reduced the age effect for neonatal and infant mortality but not for under-5 mortality in both regions (figure 4 and online supplemental figure 4). However, for stillbirths, the risk gradient versus age was stronger after adjustment for health-seeking, suggesting that some health-seeking recorded in the survey could be related to pregnancy complications or even to the stillbirths themselves. It should be emphasised that the dataset used in figure 4 and online supplemental figure S4 is smaller than the one used in figures 1–3 since the healthcare variables were available only for the last birth in the 3/5 years preceding the survey. Further adjustment for wealth quintile did not modify the age effect significantly (Model 3, online supplemental figure 3). For the 2009–2013 time period in SSA, similar patterns were observed (online supplemental figures 5–9), and in South Asia where this time period had a particularly small sample size, the age effect was reduced. For the 2004–2008 time period, the risk gradient versus age appeared in both regions for the majority of child survival outcomes (online supplemental figures 10–14).
Figure 2.
Ratios associated with neonate, infant, child, 1–59 months, under-5 years and stillbirth in SSA and South Asia for the 2014–2018 survey period. Risk factors reducing the probability of death have ORs lower than 1 to the left of the vertical red line. ORs (blue points) and 95% CIs (horizontal blue lines) are given. P values are shown with the asterisk signs (***0.001; **0.01; *0.05; ‘.’ 0.1 ‘ ’ 1). Reference group is mothers aged 23–25 years old (Model 0). SSA, Sub-Saharan Africa.
Figure 3.
Ratios associated with neonate, infant, child, 1–59 months, under-5 years and stillbirth in SSA and South Asia for the 2014–2018 survey period. Risk factors reducing the probability of death have ORs lower than 1 to the left of the vertical red line. ORs (blue points) and 95% CIs (horizontal blue lines) are given. P values are shown with the asterisk signs (***0.001; **0.01; * 0.05; ‘.’ 0.1 ‘ ’ 1). Reference group is mothers aged 23–25 years old who live in rural areas and have no formal education (Model 1). SSA, Sub-Saharan Africa.
Figure 4.
Ratios associated with neonate, infant, child, 1–59 months, under-5 years and stillbirth in SSA and South Asia for the 2014–2018 survey period. Risk factors reducing the probability of death have ORs lower than 1 to the left of the vertical red line. ORs (blue points) and 95% CIs (horizontal blue lines) are given. P values are shown with the asterisk signs (‘**0.001; **0.01; * 0.05; ‘.’ 0.1 ‘ ’ 1). Reference group is mothers aged 23–25 years old who delivered at home, and had no ANC visit (Model 2). ANC, antenatal care; SSA, Sub-Saharan Africa.
Discussion
Among young mothers in SSA and South Asia, there was a consistent risk gradient versus maternal age at all stages of child mortality and all survey periods. Compared with other known risk factors, young maternal age appears to be among the strongest risk factors of child mortality. Our findings confirm and substantially expand the conclusions from previous studies about the association between early childbearing and adverse child health outcomes,2 23 24 and suggest that the increased risk to children of younger mothers continues to linger even in regions with dropping adolescent pregnancy rates such as South Asia. Even in adjusted analyses, after controlling for several risk factors, the associations between adolescent pregnancy and child survival remained similar, except for neonatal and infant mortality where the effect was reduced after adjustment for health-seeking variables. This suggests that ensuring young mothers receive quality maternal care could reduce some of the early childbearing effects. Provision of antenatal and postnatal health services to adolescents can further be improved by recognising their biological and social needs and vulnerabilities,25 considering that adolescents may experience social stigma from healthcare providers, besides the socioeconomic limitations they deal with.26 The overall risk of death is higher among neonates, infants and children of mothers in the poorest wealth quintile living in rural areas with no formal education, across all age groups. The heterogenous and limited progress in reducing adolescent pregnancies among these vulnerable groups emphasises the inequity as well as inadequate distribution of resources and health services.12 27 However, the risk trend among younger mothers was evident across all socioeconomic status (SES) groups, suggesting that beyond external social factors placing children of younger mothers at a higher level of disadvantage than other ages, underlying biological or behavioural immaturity of the mother was likely also at play.16 27 Past studies have found that coinciding pregnancy with growth in young adolescents may lead to maternal–fetal competition for nutrients and consequently, to increased risk of low birth weight, neonatal mortality and preterm delivery.28 29
The high mortality risk of children under-5 highlights the likely long-term impact of limited education and livelihood opportunities for adolescent mothers.30 This can initiate a poverty cycle in their families, in addition to mental health and psychological challenges from social stigma that young mothers may deal with.31 32 To reduce neonatal and under-5 mortality rates towards Sustainable Development Goals (SDG) targets, it is necessary to focus efforts on reducing unintended pregnancies in adolescents in countries where they are prevalent, and ensure adolescent girls and young women have access to sexual and reproductive health services to both increase choice and regulate spacing in their fertility decisions. Our analyses highlight where the risk is the highest and where we need to learn more in order to ensure girls, young women, and their children have more favourable health outcomes.
Previous studies have found strong associations between adolescent motherhood and child or early marriage, particularly in African and Asian contexts where marriage usually precedes childbearing.18 Moreover, early marriage before age 18 has been positively associated with higher fertility, poorer maternal and reproductive health and poorer health and developmental outcomes among their children, through pathways including biological factors, social risks and maternal behaviour.31 32 Despite existing laws and human rights frameworks calling to eliminate marriage of girls before age 18, 650 million girls and women alive today married before their 18th birthday; 40% of those women live in South Asia, while 18% live in SSA.33 Strengthening measures to delay age at marriage may help reduce adolescent pregnancies in regions where both are strongly linked.
Our work has some limitations. There is likely under-reporting of mortality at early stages of child life, especially neonatal death and stillbirth. Further, survey responses rely on recall data,2 and respondents may overstate their ages at births during the interview due to social pressure. In addition, the survey data represent the sociodemographic characteristics of respondents at the time of interview, and not during the birth events. Besides the risk factors we accounted for in our study, other covariates related to the pregnancy-induced complications such as maternal nutrition status,18 which are not available in the DHS datasets, could help better explain the observed patterns. In addition, for the children under-5 mortality endpoint, other risk factors such as infectious diseases along with preterm birth complications, birth asphyxia and trauma, and congenital anomalies which are the leading causes of death for children under-5, were not included in this analysis due to lack of data.34 The limited sample size of the group suffering the greatest disparities, under-16 mothers, constrained the number of variables we could consider in any single model, particularly in South Asia, and even more so with respect to health-seeking variables. The limited sample size also required us to group all mothers under 16 in the main analysis. In sensitivity analysis, we divided the under-16 age group into two roughly equal groups of 10–14 and 15 years old in SSA for the unadjusted Model 0 as well as Model 1 and the period 2014–2018 (online supplemental figures 15 and 16). Results showed a strong age effect for all child health outcomes with children of 10–14 years old mothers doing even worse than the combined under-16 group. However, the small sample size did not allow for further analysis. Similarly, after examining the maternal mortality DHS module, we decided to not include maternal mortality in our analysis, due to the small sample size and lack of risk factors in this module, whose data are based on interviews with siblings of deceased mothers. All the abovementioned limitations in retrospective analyses of cross-sectional survey data restrain the ability to disentangle the underlying biological, behavioural and environmental mechanisms, and to rule out residual confounding factors. There is a need for longitudinal studies and follow-up data in diverse contexts to help tease apart the drivers of adverse child outcomes for young mothers.
Conclusions
Our study highlights the strong differences in child health outcomes within the under-20 maternal age group, and provides quantitative evidence on the necessity for age-disaggregated reporting and survey data on adolescent pregnancy, given the specific biological and social risks to adolescent mothers and their babies,25 to better understand its associations with child outcomes and how their nature, scale and impact vary by age. Revising the future classification of maternal age, and reporting of adolescent reproductive health will help better develop and monitor the progress of age-specific programmes aimed at achieving the SDGs of reducing adolescent pregnancy.35 By building on previous studies and policies, our work is more cognizant of empirical and health-seeking contexts, and suggests the path forward with respect to policy modification, while recognising that adolescents biological and social needs and vulnerabilities should be accounted for when improving health services and developing age-specific policies. Building the capacity of adolescents to make their own decision and choices on their reproductive health, which is shaped by numerous social, cultural and economic circumstances,36 is vital. Some of the contributing factors to this problem are beliefs, attitudes and norms in the community as well as healthcare providers about adolescent sexuality that put them at risk for poor health outcomes.26 Among women who would want to avoid pregnancy in LMICs, the unmet need for modern contraception is much higher for adolescents than for all women aged 15–49. About 44% of adolescent women in LMICs who want to avoid pregnancy have an unmet need for modern contraception.36 Interventions focused on expanding contraceptive access and use are key towards shifting social and gender norms at family and community levels,31 addressing early pregnancy, and subsequently improving child outcomes.37 Some age-specific programmes could be related to increasing awareness on the importance of interventions, laws and enforcement, and advocacy and outreach addressing individual and community barriers to delaying first pregnancy, including delaying marriage through establishing and enforcing laws,38 addressing underlying social and economic drivers and norms, empowering young women to choose if, when, and whom they marry, and enabling young women to continue and attain higher levels of education and to reduce unintended adolescent pregnancies as well as rapid repeat pregnancies.
Acknowledgments
We would like to thank Dr Guillaume Chabot-Couture and Dr Edward Wenger for their useful and constructive comments and insights.
Footnotes
Handling editor: Seye Abimbola
Contributors: JLP and APO helped develop the research concept and approach. NN analysed the data and wrote the initial draft of the manuscript. YE and JLP provided subject-matter expertise and guidance. All authors contributed to the interpretation of results and writing of the manuscript and have read and approved the final manuscript. NN is responsible for the overall content of the manuscript as the guarantor.
Funding: This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was based on secondary analysis of Demographic and Health Surveys (DHS) data. The ethical clearance was provided by the Institutional Review Board of ICF International. Therefore, this secondary analysis was exempt from ethical review approval, since it used publicly available, deidentified data. Participants gave informed consent to participate in the study before taking part.
References
- 1.WHO . Adolescent pregnancy-WHO. Available: https://www.who.int/news-room/fact-sheets/detail/adolescent-pregnancy [Accessed 03 Feb 2021].
- 2.Neal S, Channon AA, Chintsanya J. The impact of young maternal age at birth on neonatal mortality: evidence from 45 low and middle income countries. PLoS One 2018;13:e0195731–16. 10.1371/journal.pone.0195731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF . Early childbearing – UNICEF data. Available: https://data.unicef.org/topic/child-health/adolescent-health/#:~:text=Globally%2C%20almost%20one%20in%20six,age%2018%20from%202015%2D2020 [Accessed 03 Feb 2021].
- 4.Huda MM, O'Flaherty M, Finlay JE, et al. Time trends and sociodemographic inequalities in the prevalence of adolescent motherhood in 74 low-income and middle-income countries: a population-based study. Lancet Child Adolesc Health 2021;5:26–36. 10.1016/S2352-4642(20)30311-4 [DOI] [PubMed] [Google Scholar]
- 5.Sagalova V, Le Dain A-S, Bärnighausen T, et al. Does early childbearing affect utilization of antenatal care services and infant birth weight: evidence from West and central African region. J Glob Health 2021;11:13003. 10.7189/jogh.11.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mekonnen T, Dune T, Perz J. Maternal health service utilisation of adolescent women in sub-Saharan Africa: a systematic scoping review. BMC Pregnancy Childbirth 2019;19:366. 10.1186/s12884-019-2501-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yakubu I, Salisu WJ. Determinants of adolescent pregnancy in sub-Saharan Africa: a systematic review. Reprod Health 2018;15:1–11. 10.1186/s12978-018-0460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loaiza E, Liang M. Adolescent pregnancy: a review of the evidence. New York: UNFPA, 2013. [Google Scholar]
- 9.Chen X-K, Wen SW, Fleming N, et al. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol 2007;36:368–73. 10.1093/ije/dyl284 [DOI] [PubMed] [Google Scholar]
- 10.GBD 2017 Child and Adolescent Health Collaborators, Reiner RC, Olsen HE, et al. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the global burden of diseases, injuries, and risk factors 2017 study. JAMA Pediatr 2019;173:e190337. 10.1001/jamapediatrics.2019.0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal S, Channon AA, Chandra-Mouli V, et al. Trends in adolescent first births in sub-Saharan Africa: a tale of increasing inequity? Int J Equity Health 2020;19:1–11. 10.1186/s12939-020-01251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patton GC, Olsson CA, Skirbekk V, et al. Adolescence and the next generation. Nature 2018;554:458–66. 10.1038/nature25759 [DOI] [PubMed] [Google Scholar]
- 13.Finlay JE, Özaltin E, Canning D. The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: evidence from 55 low- and middle-income countries. BMJ Open 2011;1:e000226. 10.1136/bmjopen-2011-000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finlay J, Norton M, Mejía-Guevara I. Adolescent fertility and child health: the interaction of maternal age, parity and birth intervals in determining child health outcomes. Int J Child Health Nutr 2017;6:16–33. 10.6000/1929-4247.2017.06.01.2 [DOI] [Google Scholar]
- 15.Fall CHD, Sachdev HS, Osmond C, et al. Association between maternal age at childbirth and child and adult outcomes in the offspring: a prospective study in five low-income and middle-income countries (cohorts collaboration). Lancet Glob Health 2015;3:e366–77. 10.1016/S2214-109X(15)00038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Althabe F, Moore JL, Gibbons L, et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reprod Health 2015;12:1–9. 10.1186/1742-4755-12-S2-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and childbirth outcomes among adolescent mothers: a world Health organization multicountry study. BJOG 2014;121 Suppl 1:40–8. 10.1111/1471-0528.12630 [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Zhao M, Liang Y, et al. Maternal age at birth and neonatal mortality: associations from 67 low-income and middle-income countries. Paediatr Perinat Epidemiol 2021;35:318–27. 10.1111/ppe.12734 [DOI] [PubMed] [Google Scholar]
- 19.Grønvik T, Fossgard Sandøy I. Complications associated with adolescent childbearing in sub-Saharan Africa: a systematic literature review and meta-analysis. PLoS One 2018;13:e0204327. 10.1371/journal.pone.0204327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ICF . Demographic and Health Surveys (various) [Datasets]. Funded by USAID. Rockville, Maryland: ICF [Distributor], 2004-2018. [Google Scholar]
- 21.Croft TN, Marshall AMJ, Allen CK. Guide to DHS statistics. Rockville, Maryland, USA: ICF, 2018. [Google Scholar]
- 22.R Core Team . A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013. https://www.r-project.org/ [Google Scholar]
- 23.Oyeyemi AL, Aliyu SU, Sa’ad F, et al. Association between adolescent motherhood and maternal and child health indices in Maiduguri, Nigeria: a community-based cross-sectional study. BMJ Open 2019;9:e024017–9. 10.1136/bmjopen-2018-024017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozuki N, Lee ACC, Silveira MF, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health 2013;13 Suppl 3:S2. 10.1186/1471-2458-13-S3-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benova L, Neal S, Radovich EG, et al. Using three indicators to understand the parity-specific contribution of adolescent childbearing to all births. BMJ Glob Health 2018;3:e001059. 10.1136/bmjgh-2018-001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathfinder international . Beyond bias, project overview, Burkina Faso, Pakistan, Tanzania. Available: https://www.pathfinder.org/wp-content/uploads/2019/01/Beyond-Bias-Brief-2019.pdf
- 27.Saloojee H, Coovadia H. Maternal age matters: for a lifetime, or longer. Lancet Glob Health 2015;3:e342–3. 10.1016/S2214-109X(15)00034-0 [DOI] [PubMed] [Google Scholar]
- 28.Yu SH, Mason J, Crum J, et al. Differential effects of young maternal age on child growth. Glob Health Action 2016;9:31171. 10.3402/gha.v9.31171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace JM. Competition for nutrients in pregnant adolescents: consequences for maternal, conceptus and offspring endocrine systems. J Endocrinol 2019;242:T1–19. 10.1530/JOE-18-0670 [DOI] [PubMed] [Google Scholar]
- 30.Ayanaw Habitu Y, Yalew A, Azale Bisetegn T. Prevalence and factors associated with teenage pregnancy, northeast Ethiopia, 2017: a cross-sectional study. J Pregnancy 2018;2018:1–7. 10.1155/2018/1714527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efevbera Y, Bhabha J, Farmer PE, et al. Girl child marriage as a risk factor for early childhood development and stunting. Soc Sci Med 2017;185:91–101. 10.1016/j.socscimed.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 32.Wooden Q, Ch M, Nayihouba A. Economic impacts of child marriage: global synthesis report, 2017. [Google Scholar]
- 33.UNICEF . Child marriage, latest trends and future prospects. Available: https://data.unicef.org/resources/child-marriage-latest-trends-and-future-prospects/ [Accessed 01 Apr 2021].
- 34.WHO . Child mortality and causes of death. Available: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/child-mortality-and-causes-of-death [Accessed 08 Mar 2022].
- 35.Kassa GM, Arowojolu AO, Odukogbe AA, et al. Prevalence and determinants of adolescent pregnancy in Africa: a systematic review and meta-analysis. Reprod Health 2018;15:1–17. 10.1186/s12978-018-0640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sully EA, Biddlecom A, Darroch JE. Adding it up: investing in sexual and reproductive health 2019. Guttmacher institute, 2020. [Google Scholar]
- 37.Speizer IS, Calhoun LM, Her CLM. Her, His, and their fertility desires and contraceptive behaviours: a focus on young couples in six countries. Glob Public Health 2021;46:1–17. 10.1080/17441692.2021.1922732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maswikwa B, Richter L, Kaufman J, et al. Minimum marriage age laws and the prevalence of child marriage and adolescent birth: evidence from sub-Saharan Africa. Int Perspect Sex Reprod Health 2015;41:58–68. 10.1363/4105815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007681supp001.pdf (5.1MB, pdf)
Data Availability Statement
Data are available in a public, open access repository.