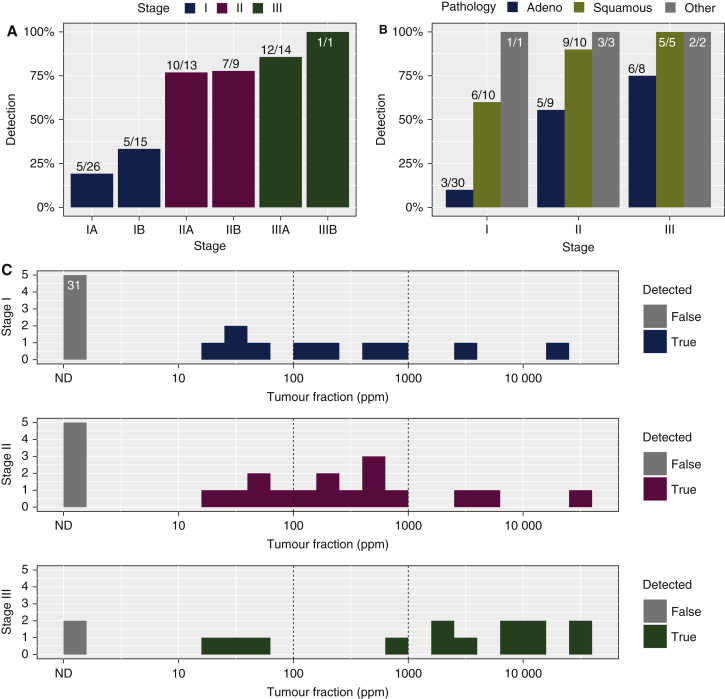
Figure 2.
ctDNA detection before treatment.
Histograms showing detection rates of ctDNA before treatment of 78 patients where plasma samples were available pretreatment, (A) according to disease stage and (B) according to disease stage and histological subtype. Detection rates are shown in percentages, and the numbers of samples in each group are indicated above the bars (detected/total). (C) ctDNA levels, shown as estimated variant allele fraction (eVAF) in parts per million (ppm) of tumour DNA to total DNA, in plasma samples collected before treatment from patients with non-small cell lung cancer at stage I (top), II (middle) and III (bottom). Samples with ctDNA not detected are shown in grey bars, including 31 samples from patients with stage I in which ctDNA was not detected before treatment. Vertical dotted lines indicate eVAF of 0.01% (100 ppm) and 0.1% (1000 ppm).
ctDNA, circulating tumour DNA; ND, not detected.