Figure 6.
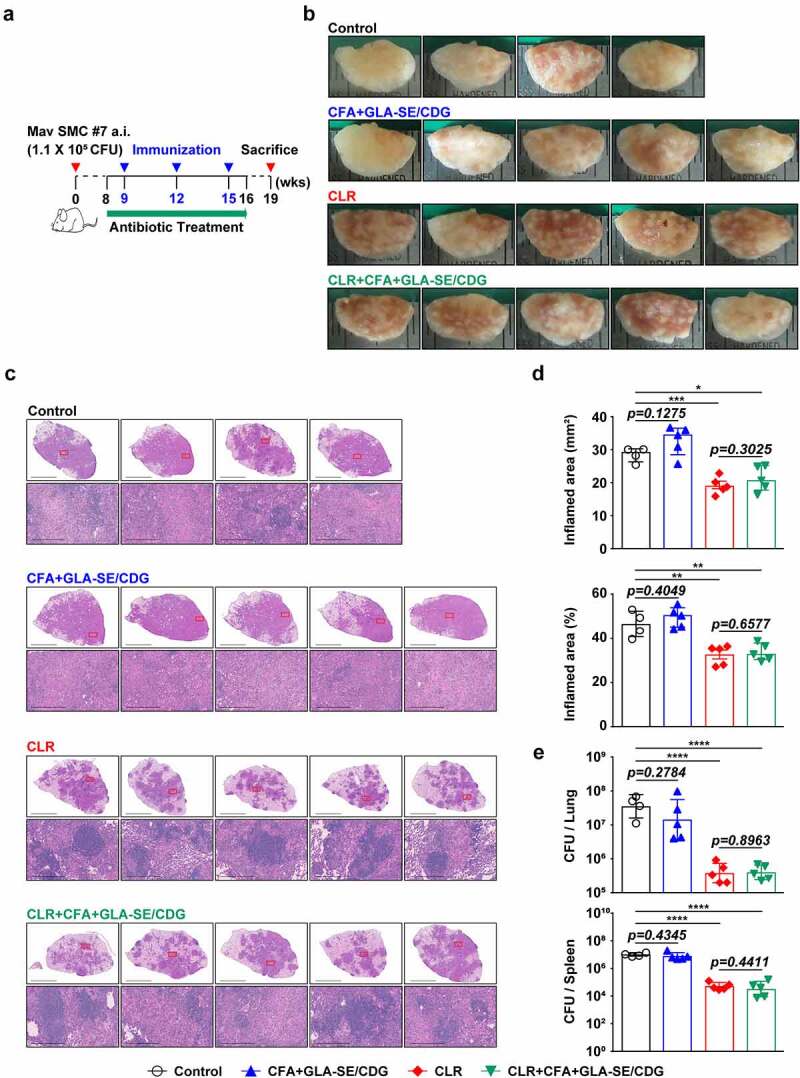
Comparative assessment of the protective efficacies of antibiotic treatment and therapeutic vaccination in a murine model of chronic progressive Mav-PI at 19 weeks post-infection. (a) Scheme of the in vivo experiment. At eight weeks after BALB/c infection with Mav SMC #7, eight weeks of daily therapy was initiated with 100 mg/kg (oral) CLR, along with immunization with CFA+GLA-SE/CDG three times at 3-week intervals. Three weeks after discontinuation of the medication, five mice in each treatment group (n = 5) and four mice in the infection control group were sacrificed, and their lungs were homogenized for bacterial CFU, lung inflammation and immunological assays at 19 weeks post-infection. (b) Gross images and (c) 10× and 200× magnification photomicrographs with H&E staining (scale bar = 3 mm and 200 µm) of the right superior lobe of infected lung tissues of all groups are displayed. Each treatment group is indicated in the upper part of the representative lung pathology image in (b) and (c). (d) Quantitative analysis of the inflamed areas in the H&E-stained lung tissues. The sizes and percentages of the lesions in (c) and the data are presented as scatter plots with bars. The bacterial burdens (e) in the left lung lobe and half of the spleen in each group were assessed by counting viable bacterial colonies grown on 7H10-OADC agar plates, and the data are presented as a scatter plot with bars. Statistically significant differences among all groups in (d) and (e) were calculated by the unpaired t test, and the results are represented as the mean values along with the S.Ds. *p <.05, **p <.01, ***p <.001, and ****p <.0001. The representative results are shown from a single in vivo experiment. CLR, clarithromycin; Control, untreated infection control; CFA, culture filtrate antigen; GLA-SE/CDG, glucopyranosyl lipid a adjuvant formulated in a stable oil-in-water emulsion plus cyclic-di-GMP.
